Shedding light on the molecular determinants of response to anti-PD-1 therapy
Progress in the understanding of the role of the immune system in tumor immunosurveillance has led to the recognition that tumors can evade immune destruction via the dysregulation of co-inhibitory or checkpoint signals. Lung tumorigenesis is not only dependent on genetic aberrants within cancer cells but also on interactions with the immune system (1). In the normal physiological state, programmed death-1 (PD-1), an immune checkpoint or co-inhibitory molecule found on activated T cells, acts to prevent autoimmunity (2). The binding of PD-1 with one of its ligands, programmed death-ligand 1 (PD-L1) (or CD274, B7-H1) or PD-L2 (CD 273, B7-DC), leads to the down-regulation of cytotoxic T cell function (2). However tumors can co-opt the PD-1/PD-L1 pathway to evade T-cell–induced antitumor response (2,3). The inhibition of the PD-1/PD-L1 pathway with immune checkpoint inhibitors can interrupt the engagement of PD-1 with its ligands and block inhibitory signals in T cells, resulting in tumor recognition by cytotoxic T cells.
The development of immune checkpoint inhibitors such as nivolumab, pembrolizumab (PD-1 inhibitors) and MPDL3280A (PD-L1 inhibitors) represent an important breakthrough in the treatment of cancer. Early phase studies of PD-1/PD-L1 inhibitors have reported impressive clinical activity and durable responses in patients with refractory tumors including melanoma, renal cell cancer, Hodgkin’s lymphoma, bladder cancer, and non-small cell lung cancer (NSCLC) (4-10) and recent phase III studies have reported pembrolizumab and nivolumab conferred OS benefit in melanoma versus ipilimumab (11) and dacarbazine (12) respectively and improvement in OS in patients with advanced stage NSCLC treated with second line nivolumab versus docetaxel (13,14).
As clinical efficacy is seen only in a subset of patients with advanced stage NSCLC treated with PD-1/PD-L1 inhibitors, biomarkers are needed to improve patient selection for immune checkpoint inhibition. Whilst histologic subtype was not associated with response, current or former smoking status has been reported to be associated with an increased response to treatment in several studies (Table 1). A possible explanation for this finding is smoking-associated lung cancers have a higher mutational load, resulting in the creation of more tumor neoantigens and increased immunogenicity (16,17).
Since the PD-1/PD-L1 pathway is implicated in immune escape in NSCLC, tumor PD-L1 expression with immunohistochemistry (IHC) has been used as a predictive biomarker. In one study, no association between tumor PD-L1 expression and response to nivolumab was found (15) whereas in KEYNOTE 001, tumor PD-L1 expression was associated with response to pembrolizumab (10). A possible confounder was PD-L1 expression was determined using archival tumor samples in the nivolumab study whereas a fresh tumor biopsy was obtained in the latter study.
The development of anti-PD-L1 IHC companion diagnostics by several pharmaceutical companies has created some challenges as these assays are using different IHC antibody clones, different staining protocols and platforms, different scoring systems, and different cutoffs defining positivity. Other issues influencing PD-L1 assessment include tumor heterogeneity, the dynamic nature of PD-L1 expression (18-20) and differences in the assessment of PD-L1 in the tumor microenvironment (tumor cell, stroma, or both) (Table 2). Regarding the latter factor, PD-L1 expression in the tumor microenvironment may be an important determinant of response. Herbst and colleagues found PD-L1 expression in tumor infiltrating immune cells, instead of tumor cells, was associated with response in patients with advanced stage NSCLC treated with MPDL3280A (8) whereas immune cells at the invasive margin expressing PD-1 and PD-L1 was associated with response in patients with advanced stage melanoma treated with pembrolizumab (19). Furthermore, whilst high PD-L1 expression is associated with a higher response rate, responses of 5-20% are also seen in PD-L1 negative patients, adding further complexity to using PD-L1 as a biomarker (Table 2).
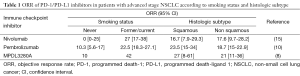
Full table

Full table
As immune checkpoint inhibitors are active in bladder cancer (6), NSCLC (8,10,15), and melanoma (11,12), tumors typically associated with high somatic mutation rates (16), it has been hypothesized that the mutational landscape is a determinant of response to PD-1/PD-L1 inhibitors. Based on this premise, investigators have recently reported mutational burden in NSCLC was associated with response to PD-1 inhibition. In this seminal Science paper, the subject of this editorial, Rizvi and colleagues performed whole-exome sequencing of tumors from a phase I study of patients with advanced stage NSCLC treated with pembrolizumab (23). In two independent patient cohorts they found a high somatic nonsynonymous mutation burden was associated with greater durable clinical benefit (defined as a partial or stable response of at least 6 months), higher objective response rates and a longer progression free survival. In addition, clinical efficacy was associated with a molecular smoking signature, certain DNA repair mutations and the burden of neoantigens. Interestingly the molecular smoking signature, rather than self-reported smoking status, correlated with efficacy.
Furthermore, a high nonsynonymous mutational burden correlated with a greater number of putative neoantigens with high binding affinity to patient-specific HLA alleles, and patients who had a durable clinical benefit had a higher neoantigen burden than those who did not. These findings are significant as they support the hypothesis that the recognition of neoantigens, formed as a result of somatic mutations, is important for anti PD-1 activity and provides further proof of principle that tumor genomics can dictate responses to immunotherapy. The same investigators have recently reported mutational load and a neoantigen landscape that is specifically present in melanomas was associated with response to CTLA-4 inhibition (17). Another notable finding is T-cell response against a mutation-associated neoantigen was detected in peripheral blood lymphocytes from one patient who responded to pembrolizumab. This important finding creates an opportunity to develop blood-based assays to monitor response to PD-1 inhibition.
What is unknown from this study is the correlation between mutational load and PD-L1 density and more studies are needed to determine the relationship between mutational burden and PD-L1 expression. Further studies are also required to confirm these findings with other PD-1/PD-L1 inhibitors and in other malignancies. Research in identifying mechanisms of resistance to immune checkpoint inhibitors will also aid in the development of rational therapeutic strategies and ultimately improve patient outcomes. As PD-L1 IHC assays may be introduced into clinical use in the near future, the International Association for the Study of Lung Cancer is planning an international characterization study of PD-L1 companion diagnostics to gain a better understanding of the assays, their performance on different sample types (large specimens, small biopsies, cytology) and different on platforms.
In summary, through a better understanding of the molecular determinants of response to immunotherapy, Rizvi et al. has provided insight on how tumor mutational load in NSCLC influence the efficacy of anti-PD-1 therapy and T-cell responses to neoantigens created by somatic mutations might underpin pembrolizumab activity in NSCLC. The results also underscore the importance of incorporating biomarkers in studies to characterize molecular mechanisms of sensitivity.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Hongbing Liu (Department of Respiratory Medicine, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol 2015.33.
- Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 2015.33.
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [PubMed]
- Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101-9. [PubMed]
- Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 2014;32:5s.
- Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32:8021.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [PubMed]


