Lung cancer in HIV-infected patients in the combination antiretroviral treatment era
Introduction
The introduction of combination antiretroviral treatment (cART) has been followed by a dramatic decrease in the morbidity and mortality associated with HIV-infection, including the incidence of AIDS-defining cancers (1). At the same time, however, the incidence of various non-AIDS-defining cancers (NADCs) seems to have risen (2-6). These so-called NADCs consist of a heterogeneous group of malignancies including cancers of the lung, liver, kidneys, anus, head and neck, and skin, and Hodgkin’s lymphoma, among others, and make concurrent treatment with antineoplastic agents and cART necessary.
Increased life expectancy and the reduction of competing causes of death are both driving the increased incidence of NADCs in the HIV-infected population. Other contributors may also include the effects of HIV itself, the greater prevalence of co-infection with oncogenic viruses [e.g., human herpesvirus 8, human papilloma virus, Epstein-Barr virus (EBV), and hepatitis B and C viruses], and different environmental factors such as tobacco and alcohol use (2).
Of all the NADCs seen in HIV-infected patients, lung cancer is the most frequent, contributing to significant comorbidity in this population. This review focuses on the epidemiology, risk factors, and clinical management of lung cancer in HIV-infected patients.
Epidemiology and contributing factors
The risk of lung cancer has been estimated to be 2-7 times higher in HIV-infected patients than in the general population (2,4,7-12). Both aging and high prevalence of tobacco use in the HIV-infected population contribute to this elevated risk. Other possible risk factors of lung cancer among HIV-infected patients may also include the HIV itself, the presence of advanced immunosuppression, and chronic pulmonary inflammation.
Just as in the general population, the incidence of lung cancer increases with age in HIV-positive population. Thus, as happens with other NADCs, any apparent increase in the incidence of lung cancer in HIV-positive patients may in part simply reflect the process of normal aging of the HIV-infected population after the introduction of cART (5,9,13).
In addition, the increase in survival associated with cART may also lead to increased exposure to oncogenic factors such as viral co-infections, tobacco, alcohol, or sun exposure, which may all contribute to an increased risk of NADCs. In particular, the high prevalence of smoking among HIV-infected patients contributes to the greater risk of lung cancer in this population (1,5,9,11). Thus, most lung cancers in HIV-infected patients occur in current or former heavy smokers, while only a small proportion of lung cancers occur in patients who never smoked (14). The substantial lung cancer risk at younger ages coupled with an advanced stage at diagnosis suggests that smoking causes more severe damage in HIV-infected patients as well as an accelerated pathogenesis (8). However, it is important to note that the risk of lung cancer in HIV-infected patients remains increased heightened even after accounting for tobacco use (7), suggesting that other factors may be involved.
Besides classical risk factors, the increased risk of lung cancer in HIV-infected patients may reflect the consequences of increased immune activation and decreased immune surveillance, as well as direct effects of HIV. For example, the HIV itself may activate proto-oncogenes, cause alterations in cell cycle regulation, inhibit tumour suppressor genes, or cause genetic alterations leading to oncogenesis (15-17). Additionally, infected cells may be more sensitive to the effects of environmental carcinogens (18). On the other hand, HIV-related immunosuppression can lead to chronic immune activation, inflammation, and immune system dysfunction, which can increase the risk of developing cancer (19-21). Comparative studies have shown that the pattern of cancers in HIV-infected patients is similar to that seen in immunosuppressed transplant patients (22), and immunosuppression associated with HIV infection has been established as a risk factor for various NADCs, including lung cancer (4). However, the relationship between CD4+ T cell count or duration of immunodeficiency and lung cancer risk remains uncertain, with some studies finding an association whereas others have not (1,5,8,11,23,24).
Finally, chronic lung inflammation due to pre-existing diseases, both infectious and non-infectious, has been associated with a trend towards increased risk of lung cancer. In this regard, some authors have reported that HIV-infected individuals with recurrent pneumonia may be at a significantly higher risk of lung cancer compared with those without such a history (5,25).
Clinical characteristics
Lung cancer in HIV-infected individuals is frequently diagnosed at younger ages than in the general population (26-28), with a median age at diagnosis of 47 years compared to 70 years in the general population (29-33). In addition, the vast majority of lung cancers in HIV-infected patients are diagnosed at advanced stages, with more than half of the cases being diagnosed at stage III or IV (8,12,26,34). Adenocarcinoma is the most common histological type, followed by squamous cell carcinoma, large-cell carcinoma and small-cell lung carcinoma (5,8,12,14,35).
Traditionally, HIV infection has been associated with poorer prognosis and overall survival in patients with lung cancer, suggesting that lung cancer has a more aggressive phenotype in HIV-infected patients. Biggar et al. (36) reported a 2-year survival rate of only 10% in patients with HIV, compared with 31% in the general population. Similarly, the 5-year survival rate for lung cancer was only 10% in HIV-infected patients whereas it was 19% in HIV-negative patients in a recently published study that evaluated the survival rates for incident NADCs in 22,081 HIV-infected and 230,069 non-HIV-infected individuals (37). However, it is important to note that most of these data come from retrospective studies that grouped patients with non-small-cell and small-cell lung cancer, and that included a high number of patients from the pre-cART era. By contrast, Rengan et al. (38) found that survival rates were similar for both HIV-positive and HIV-negative patients diagnosed with non-small-cell lung cancer between 2000 and 2005. Similarly, Hakimian et al. (39) reported comparable survival rates in patients with advanced non-small-cell lung cancer between HIV-positive patients with CD4 cell counts >200 cells/mm3 and patients without HIV infection. Therefore, these data suggest that, although HIV infection could have been an adverse prognostic factor in the pre-cART era, this effect has diminished after the advent of cART.
Clinical management
Disparities in cancer treatment between HIV-infected patients and the general population may contribute to the worse prognosis of lung cancer among HIV-infected patients. Suneja et al. (28) reported that only 60.3% of HIV-infected patients with lung cancer were offered cancer treatment compared with 77.5% of HIV-negative patients. Possible reasons for this disparity include the lack of specific cancer treatment guidelines for HIV patients, the false perception of an HIV-positive individual as having a lower performance status, and the greater risk of potential drug interactions and/or drug toxicity. However, noting that in instances of lymphoma it has been shown that full doses of chemotherapy can be safely administered in combination with cART, resulting in increased survival rates, we can postulate that the same approach will be equally effective for other NADCs. In fact, several retrospective studies have suggested an acceptable toxicity profile when chemotherapy is combined with cART. As a result, there is an increasing tendency to consider exactly the same cancer treatment options for both HIV-infected people and the general population.
Nonetheless, the question of whether combining cART with chemotherapy outweighs the potential risk of increased toxicity has remained controversial. Some authors have justified postponement or interruption of cART during chemotherapy based on the risk of overlapping toxicities and the potential for difficult-to-manage drug-drug interactions between antineoplastics and antiretroviral drugs. However, early and effective cART during chemotherapy has been shown to increase survival rates in several studies (34,40,41). For example, Lavolé et al. (34) reported a 60% reduction in overall mortality associated with the use of cART in HIV-infected patients with non-small-cell lung cancer. On the other hand, interruption of cART has been associated with higher risk of death, AIDS, and serious non-AIDS morbidity (42). In light of these findings, initiation or maintenance of cART is now recommended for HIV-infected patients with cancer (43), and concomitant treatment with cART and chemotherapy is growing increasingly common.
The timing of diagnoses of HIV and malignancy may guide therapy decisions. If a patient is known to be HIV-positive and is diagnosed with a curable malignancy while he is already taking cART, all attempts should be made to achieve proper exposure of the antineoplastic drugs in order to maximize the chance of cure and, at the same time, minimize any toxicity. On the other hand, if a patient is diagnosed with both HIV infection and a malignancy at the same time, antineoplastics should be started first, followed by cART, once the patient is tolerating antineoplastic therapy (44).
There are no current guidelines to address lung cancer treatment in HIV-infected individuals, and HIV infection may be perceived as a challenge by treating oncologists. Two crucial aspects need to be taken into account in this situation. First, drug interactions that may modify the concentrations of one or several drugs are possible, potentially leading to excessive toxicity and/or decreased efficacy; and, second, there may be overlapping toxicities between antiretroviral and antineoplastic drugs that should be considered when prescribing both treatments. In such a scenario, interdisciplinary collaboration is mandatory for the optimal treatment of the oncologic process and HIV infection. The discontinuation of a single drug in the antiretroviral regimen thought to be responsible for an interaction or an overlapping toxicity with chemotherapy must be avoided, as this may decrease the efficacy of cART, as well as the temporary discontinuation of cART (45). Both oncologic and cART treatments must be tailored by oncologists and HIV specialists in order to maximize the chances of response with minimal toxicity.
Management of antineoplastic treatment
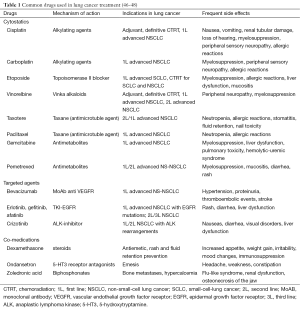
As shown in Table 1, there are several antineoplastic drugs currently available for the management of patients with lung cancer in different scenarios. Various factors need to be considered when designing an antineoplastic regimen for a patient with lung cancer. These include the presence of underlying molecular alterations which may be specifically targeted by therapy, the performance status of the patient, prior administered drugs, and toxicities related to prior treatments.
Patients harbouring tumours with mutations in the EGFR gene deserve special attention. There are some case reports of HIV-infected patients with lung cancer harbouring EGFR mutations who were treated with the EGFR-TKI erlotinib or gefitinib (49,50). Lung cancer therapy outcomes in terms of response and duration of response were good, and data were in line with prior results from the literature, providing a novel therapeutic strategy for these patients. The prevalence of EGFR mutations in HIV-infected patients with lung cancer has been found to be similar to the general population in a Japanese cohort study (51), with EGFR mutations being present in 35.7% of the patients. Thus, although specific clinical trials are still lacking, these data suggest that HIV infection should not be a reason to modify the general lung cancer work-up process. In patients with non-squamous NSCLC histology and no or light smoking history, the screening for underlying molecular alterations such as EGFR mutations and ALK translocations should be performed in order to offer the most appropriate therapy to those patients who test positive.
Drug interactions between antiretroviral and antineoplastic agents
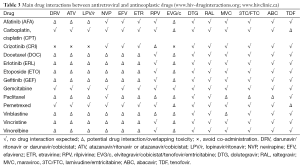
Although controlled studies have not been performed, clinically relevant interactions between chemotherapy regimens and cART have been reported in the literature. Table 2 lists antiretroviral drugs in current use according to their interaction potential with antineoplastic agents, and Table 3 summarizes main possible drug interactions between antiretrovirals and antineoplastics in common use to treat patients with lung cancer. The potential for interactions appears to be particularly high with antiretroviral regimens that include strong enzyme inhibitors such as ritonavir-boosted protease inhibitors (PIs). Ritonavir is a potent inhibitor of cytochrome P450 enzymes and drug transporters which are involved in the disposition of numerous drugs, leading to marked increases in drug exposure (52). Consequently, the use of ritonavir-boosted PIs has been shown to substantially raise the risk of adverse events in HIV-infected patients treated with antineoplastics including vinca alkaloids, taxanes, etoposide, and tyrosin kinase inhibitors (41,53-55). Moreover, Torres et al. (41) have recently reported results of a retrospective study comparing different cART regimens in 154 HIV-infected patients who received chemotherapy due to different types of cancer. In this study, PI-based regimens were the least favourable, both because of higher risk of developing adverse events and also due to lower antiviral efficacy and higher mortality rates. All these issues together with the current availability of other cART options with similar efficacy and better tolerability suggest that PI-based cART should be held in reserve as a secondary treatment option for HIV-infected patients undergoing chemotherapy.

Full table
Full table
In contrast to PIs, most NNRTIs are inducers of cytochrome P450 enzymes, and could potentially reduce the exposure, and thus efficacy, of certain chemotherapy drugs. Rilpivirine, a second-generation NNRTI, does not induce the P450 system, and theoretically its potential for interactions with chemotherapy should be much more limited (56). However, there is still little clinical data in this regard.
Integrase inhibitors are considered to be among the preferred options for cART in HIV patients with cancer due to their favourable drug interaction profile with antineoplastic drugs. Raltegravir and dolutegravir are mainly metabolized by glucuronidation through UGT1A1, and they do not have any inducer/inhibitor effect on either P450 enzymes or drug transporters, minimizing their potential for drug interactions (57,58). Conversely, elvitegravir primarily undergoes oxidative metabolism, and it needs to be co-administered with cobicistat, a new pharmacokinetic enhancer which is a moderate-to-potent inhibitor of cytochrome P450 enzymes (59). For this reason, the use of elvitegravir-based cART in HIV-infected patients receiving chemotherapy is expected to share most of the limitations of regimens that include ritonavir-boosted PIs.
Concomitant use of cART with chemotherapy may be limited not only by pharmacokinetic interactions but also by pharmacodynamic issues leading to overlapping toxicity. This may be the case of tenofovir, which can be associated with renal toxicity, making it necessary to closely monitor renal function during antineoplastic treatment, especially if the patient is receiving other nephrotoxic drugs (e.g., cisplatin, pemetrexed, etc.). Another example of overlapping toxicities may be represented by QT interval prolongation associated with both antiretroviral drugs (e.g., atazanavir, lopinavir, saquinavir, or rilpivirine) and antineoplastic agents such as tyrosine kinase inhibitors (e.g., crizotinib).
In addition to antineoplastic agents, patients with cancer often receive many other drugs (such as steroids, serotonin 5-HT3 receptor antagonists, biphosphonates, or proton pump inhibitors) to alleviate different symptoms or the side effects of chemotherapy. Since many of these co-medications may be involved in specific drug-drug interactions or may present overlapping toxicity with HIV treatment, it is highly recommended to review all the medication that the patient is taking at each medical appointment, and adjust the therapeutic plan accordingly. For example, the interaction of corticosteroids with some cART with a potent CYP3A4 activity may inhibit corticosteroid degradation and increases its accumulation. Finally, beside cART, clinicians should be aware of eventual reductions in CD4 cell count during chemotherapy. Thus, HIV-patients on antineoplastics should be monitored for CD4 cell count, and prophylaxis of opportunistic infections should be started when warranted (2).
Screening of lung cancer in the HIV-infected population
Since lung cancer contributes to substantial morbidity and mortality in both HIV-positive and HIV-negative populations, there is a growing interest in screening strategies aimed at diagnosing new cases of lung cancer at earlier stages.
The advent of low-dose helical computed tomography (LDCT) has altered the landscape of lung-cancer screening, with studies indicating that LDCT may detect many tumours at early stages. The National Lung Screening Trial randomized >50,000 current or former heavy smokers (>30 pack-year) to receive either a LDCT or a single-view posteroanterior chest radiography (CXR) annually for three consecutive years (60). Overall, the rate of positive screening tests was 24.2% with LDCT and 6.9% with CXR, and lung cancer was finally diagnosed in 3.6% and 5.5% of the participants, respectively. Interestingly, LDTC had an impact on survival, with a relative reduction in mortality from lung cancer of 20.0%. However, since the incidence of lung cancer was low, the cost-effectiveness of this strategy may be questioned, especially in the context of competing interventions such as smoking cessation programmes.
One potential drawback for the use of LDCT as a screening tool for lung cancer in HIV-infected patients may be the high number of false positive tests. A Veterans Aging Cohort Study (VACS) prospectively compared rates of abnormal findings in LDCTs performed on 160 HIV-positive and 139 HIV-negative individuals, with smoking history in 85% and 81% of the patients, respectively (61). No differences were found in terms of either abnormal findings (29% in HIV+ vs. 24% in HIV−) or lesions suspicious for cancer (4% vs. 3%). Lung cancer was diagnosed only in 3 and 1 subjects (2% vs. 0.7%), respectively. HIV-infected participants with low CD4 cell count were more likely to have a positive LDCT screening, even after adjusting for tobacco use, probably because of a higher risk of lung infections. According to these results, HIV infected patients with CD4 counts >200 do not have a higher likelihood of abnormal findings in a LDCT screening compared to uninfected controls, despite more previous lung infections and a greater risk of lung cancer.
Hulbert et al. (62) recently reported the results of the first study that prospectively evaluated the usefulness of computed tomography as a screening tool for lung cancer in a cohort of 224 HIV patients at high risk due to their heavy smoking history. The median age was 48 years, with a 34 pack-year history of smoking. During 678 person-year of follow-up, just one lung cancer was diagnosed, despite the high rate of active smoking. The authors explain this low incidence of lung cancer as probably due to the young age of the participants, and they suggest that advanced age should be recommended as an inclusion criterion in screening programs. However, this statement may be in disagreement with the clinical presentation of lung cancer at younger ages among HIV patients (26-28,30-33). Therefore, the as yet unpublished results from another study recently completed in France which evaluated the utility of LDCT for early lung cancer diagnosis in an HIV population (NCT01207986) will be of interest.
Future directions
Multidisciplinary approach
The optimal health care of a patient with simultaneous HIV infection and lung cancer may be complex, but is no more so than treating lung cancer patients with other co-morbidities, such as cardiovascular or chronic respiratory diseases, who may need polypharmacy for different underlying conditions. Currently, care decisions made by multidisciplinary teams are the preferred option when deciding how to treat a patient with lung cancer. Usually these teams consist of thoracic surgeons, pulmonary specialists, radiologists, and medical and radiotherapy oncologists. For therapeutic decisions specifically affecting patients with HIV infection, the participation of a physician with expertise in treating HIV infection is crucial. Special attention should be paid when prescribing both cART and cytostatics or molecularly targeted agents, to avoid drug interactions and overlapping toxicities that could potentially jeopardize both the efficacy of these treatments and the patient’s safety.
Including HIV-infected patients in oncology clinical trials
Patients with HIV infection have been conventionally excluded as candidates for oncology clinical trials. In particular, HIV infection is considered an exclusion criterion in 25% of the clinical trials testing new drugs/combinations to treat lung cancer (63).
Reasons to justify this exclusion include the potential risk of combining cART and cytostatics or targeted agents, the immune impairment associated with the infection, and the potential risk for lethal infections when HIV patients are treated with marrow-suppressive therapy. In line with this rationale, the NCI-CTEP states that if survival is a primary endpoint, it may be appropriate to exclude patients with known HIV-positive status for whom the probability of death due to underlying HIV-infection is higher over the likely time course of the study (64). However, nowadays this rationale is obsolete. Antiretroviral drugs currently available are safe enough to permit designing effective cART regimens with minimal drug interactions and overlapping toxicities with anticancer therapies (65,66). Moreover, with the introduction of cART the survival of HIV patients has improved, and the main factor affecting their survival is the lung cancer itself, lung cancer being one of the most common cancer-related causes of death in general and the leading cause of death in people with HIV infection after the introduction of cART in some series (67,68).
Accordingly, it may be appropriate to allow the participation of HIV-infected patients in oncology clinical trials if the HIV-infection is properly controlled. In order to offer these patients the opportunity to benefit from potentially advantageous interventions, a reasonable approach would be either to include a certain stratum of HIV-positive patients in a given clinical trial or to design specific clinical trials for this population. This way of thinking is beginning to gain acceptance, and several drugs have already been tested in HIV-infected patients with solid tumours, including lung cancer (NCT01249443, NCT02134886, NCT00890747, and NCT02408861).
Evaluating the role of PD1/PDL1 in both the HIV infection and the lung cancer
Recently, the inhibitory molecule programmed death 1 (PD-1) has gained attention in HIV research since it plays a central role in the regulatory T cell response. Dysfunctional virus-specific T- and B-cell responses are crucial for the diminished immune control in chronic infections, such as the HIV infection. PD-1 is expressed on CD4, CD8, NK T cells, B cells, and monocytes, and it has been shown to be involved in both acute and chronic HIV infection. PD-1 is upregulated in CD4, CD8, and B cells during HIV infection, leading to an exhaustion process and, consequently, to an impaired immune response. Prior studies have demonstrated that PD-1 blockade mediates the functional restoration of exhausted virus-specific CD8 in vivo, including the ability to clear viral antigens and control chronic viral infection. Similarly, impaired CD4 cells also restore their helper functions when they are exposed to PD-1 blockers, and B cells also enhance the production of virus-specific antibodies under PD-1 blockage (69).
Interestingly, recent publications have demonstrated the efficacy of PD-1 inhibition in NADCs, including lung cancer. PD-L1 and P-DL2, the PD-1 ligands, are expressed by tumour cells and tumour-infiltrating immune cells. Some antibodies against PD-1 have demonstrated a significant efficacy when PD-L1 levels are highly expressed, while others have not shown any correlation between efficacy and expression levels (70,71). In addition, when compared with standard therapies in second line, PD-1 antibody nivolumab has demonstrated a higher efficacy with less significant toxicity (72-74).
Considering these facts, interest in using PD-1 inhibitor antibodies to simultaneously treat HIV infection and NADCs has grown in the fields of both oncology and infectious disease, and this interest has crystallized in a clinical trial testing the activity of PD-1 blockers in HIV-positive patients with solid tumours (NCT02408861).
Conclusions
In conclusion, lung cancer is the most common NADC in HIV-infected patients, and it contributes to significant morbidity and mortality in this population. While the role of LDCT as a screening tool of lung cancer remains to be determined, smoking cessation strategies and early initiation of cART are strongly recommended due to the potential contribution of smoking or advanced immunosuppression to lung cancer pathogenesis. The therapeutic approach for lung cancer in HIV-infected patients should not differ from that of the general population. Although the concomitant administration of chemotherapy with cART may be challenging due to drug-drug interactions and overlapping toxicity, HIV-infected patients with lung cancer should be placed or maintained on cART during treatment. Thus, interdisciplinary collaboration between oncologists and HIV specialists is crucial for the optimal treatment of both the oncologic disease and the HIV infection while minimizing the risk of adverse outcomes for the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: J Moltó, G Sirera, and B Clotet have received research funding, consultancy fees or lecture sponsorships from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Janssen, and ViiV Healthcare. T Moran has no conflicts of interest to declare.
References
- Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005;97:425-32. [PubMed]
- Deeken JF. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012;55:1228-35. [PubMed]
- Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753-62. [PubMed]
- Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001;285:1736-45. [PubMed]
- Hessol NA, Martínez-Maza O, Levine AM, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS 2015;29:1183-93. [PubMed]
- Engels EA, Pfeiffer RM, Goedert JJ, et al. Trend in cancer risk among people with AIDS in the United States 1980-2002. AIDS 2006;20:1645-54. [PubMed]
- Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 2006;24:1383-8. [PubMed]
- Chaturvedi AK, Pfeiffer RM, Chang L, et al. Elevated risk of lung cancer among people with AIDS. AIDS 2007;21:207-13. [PubMed]
- Shiels MS, Cole SR, Kirk GD, et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009;52:611-22. [PubMed]
- Herida M, May-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol 2003;21:3447-53. [PubMed]
- Clifford GM, Lise M, Franceschi S, et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer 2012;106:447-52. [PubMed]
- Bower M, Powles T, Nelson M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS 2003;17:371-5. [PubMed]
- Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011;53:1130-9. [PubMed]
- Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26:1017-25. [PubMed]
- Wright S, Lu X, Peterlin BM. Human immunodeficiency virus type 1 tat directs transcription through alteration sites within the mouse c-myc gene. J Mol Biol 1994;243:568-73. [PubMed]
- Amini S, Khalili K, Sawaya BE. Effect of HIV-1 Vpr on cell cycle regulators. DNA Cell Biol 2004;23:249-60. [PubMed]
- Li CJ, Wang C, Friedman DJ, et al. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proc Natl Acad Sci USA 1995;92:5461-4. [PubMed]
- Altavilla G, Caputo A, Lanfredi M, et al. Enhancement of chemical hepatocarcinogenesis by the HIV-1 tat gene. Am J Pathol 2000;157:1081-9. [PubMed]
- Kirk GD, Merlo C, O'Driscoll P, et al. HIV infection is associated with an increased risk of lung cancer, independent of smoking. Clin Infect Dis 2007;45:103-10. [PubMed]
- Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl cancer Inst 2011;103:1112-22. [PubMed]
- Dubrow R, Silvenberg MJ, Park LS, et al. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol 2012;24:506-16. [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [PubMed]
- Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009;10:1152-9. [PubMed]
- Mbulaiteye SM, Biggar RJ, Goedert JJ, et al. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr 2003;32:527-33. [PubMed]
- Shebl FM, Engels EA, Goedert JJ, et al. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr 2010;55:375-9. [PubMed]
- Lavolé A, Wislez M, Antoine M, et al. Lung cancer, a new challenge in the HIV-infected population. Lung Cancer 2006;51:1-11. [PubMed]
- Pakkala S, Ramalingman SS. Lung cancer in HIV-positive patients. J Thorac Oncol 2010;5:1864-71. [PubMed]
- Suneja G, Shiels MS, Melville SK, et al. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS 2013;27:459-68. [PubMed]
- Worm SW, Bower M, Reiss P, et al. Non-AIDS defining cancers in the D:A:D Study - time trends and predictors of survival: a cohort study. BMC Infect Dis 2013;13:471. [PubMed]
- Tirelli U, Spina M, Sandri S, et al. Lung carcinoma in 36 patients with human immunodeficiency virus infection. The Italian Cooperative Group on AIDS and Tumors. Cancer 2000;88:563-9. [PubMed]
- Lavole A, Massiani MA, Wislez M, et al. Lung cancer in HIV-infected patients in the era of HAART: a case control study focusing on prognostic factors of survival. Lung Cancer 2003;41:S3-14.
- Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg 1997;84:1068-71. [PubMed]
- Spano JP, Massiani MA, Bentata M, et al. Lung cancer in patients with HIV Infection and review of the literature. Med Oncol 2004;21:109-15. [PubMed]
- Lavolé A, Chouaid C, Baudrin L, et al. Effect of highly active antiretroviral therapy on survival of HIV-infected patients with non-small-cell lung cancer. Lung Cancer 2009;65:345-50. [PubMed]
- Bearz A, Vaccher E, Martellotta F, et al. Lung cancer in HIV patients: the GICAT experience. Eur Rev Med Pharmacol Sci 2014;18:500-8. [PubMed]
- Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr 2005;39:293-9. [PubMed]
- Marcus JL, Chao C, Leyden WA, et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev 2015;24:1167-73. [PubMed]
- Rengan R, Mitra N, Liao K, et al. Effect of HIV on survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: a population-based study. Lancet Oncol 2012;13:1203-9. [PubMed]
- Hakimian R, Fang H, Thomas L, et al. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol 2007;2:268-72. [PubMed]
- Navarro JT, Ribera JM, Oriol A, et al. Favourable impact of virological response to highly active antiretroviral therapy on survival in patients with AIDS-related lymphoma. Leuk Lymphoma 2002;43:1837-42. [PubMed]
- Torres HA, Rallapalli V, Saxena A, et al. Efficacy and safety of antiretrovirals in HIV-infected patients with cancer. Clin Microbiol Infect 2014;20:O672-9. [PubMed]
- El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283-96. [PubMed]
- Torres HA, Mulanovich V. Management of HIV infection in patients with cancer receiving chemotherapy. Clin Infect Dis 2014;59:106-14. [PubMed]
- Beumer JH, Venkataramanan R, Rudek MA. Pharmacotherapy in cancer patients with HIV/AIDS. Clin Pharmacol Ther 2014;95:370-2. [PubMed]
- Silverberg MJ, Neuhaus J, Boer M, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS 2007;21:1957-63. [PubMed]
- DeVita VT, Lawrence TS, Rosenberg SA, et al., editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 8th Edition. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins, 2008:385-548.
- Chabner BA, Lynch TJ, Longo DL, et al., editors. Harrison's Manual of Oncology. New York: McGraw-Hill Companies Inc., 2008.
- National Comprehensive Cancer Network guidelines for Non-Small-Cell Lung Cancer Management. Available online: www.nccn.org/professionals/physician_gls/PDF/nscl.pdf
- Okuma Y, Hosomi Y, Imamura A. Lung cancer patients harboring epidermal growth factor receptor mutation among those infected by human immunodeficiency virus. Onco Targets Ther 2015;8:111-5. [PubMed]
- Erickson TM, Koeppe JR, Miller YE, et al. Brochioloalveolar carcinoma presenting as chronic progressive pulmonary infiltrates in a woman with HIV: a diagnosis worth making. J Thorac Oncol 2008;3:1353-5. [PubMed]
- Okuma Y, Tanura J, Kamiryo H, et al. A multi-institutional study of clinicopathological features and molecular epidemiology of epidermal growth factor receptor mutations in lung cancer patients living with human immunodeficiency virus infection. J Cancer Res Clin Oncol 2015;141:1669-78. [PubMed]
- King JR, Wynn H, Brundage R, et al. Pharmacokinetic enhancement of protease inhibitor therapy. Clin Pharmacokinet 2004;43:291-310. [PubMed]
- Bower M, Powels T, Stebbing J, et al. Potential antiretroviral drug interactions with cyclophosphamide, doxirrubicin, and etoposide. J Clin Oncol 2005;23:1328-9. [PubMed]
- Levêque D, Santucci R, Pavillet J, et al. Paralytic ileus possibly associated with interaction between ritonavir/lopinavir and vincristine. Pharm World Sci 2009;31:619-21. [PubMed]
- Moltó J, Rajoli R, Back D, et al. Physiologically based pharmacokinetic model to predict drug-drug interaction in patients receiving antiretroviral and antineoplastic therapies. 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Threrapy. Alexandria, VA, May 2015.
- Sharma M, Saravolatz LD. Rilpivirine: a new non-nucleoside reverse transcriptase inhibitor. J Antimicrob Chemother 2013;68:250-6. [PubMed]
- Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013;52:981-94. [PubMed]
- Liedtke MD, Tomlin CR, Lockhart SM, et al. Long-term efficacy and safety of raltegravir in the management of HIV infection. Infect Drug Resist 2014;7:73-84. [PubMed]
- Shah BM, Schafer JJ, Priano J, et al. Cobicistat: a new boost for the treatment of human immunodeficiency virus infection. Pharmacotherapy 2013;33:1107-16. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395-409. [PubMed]
- Sigel K, Wisnivesky J. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS 2014;28:1007-14. [PubMed]
- Hulbert A, Hooker CM, Keruly JC, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol 2014;9:752-9. [PubMed]
- Persad GC, Little RF, Grady C. Including persons with HIV infection and cancer in clinical trials. J Clin Oncol 2008;26:1027-32. [PubMed]
- National Cancer Institute Cancer Therapy Evaluation Program: Guidelines regarding the inclusion of cancer survivors and HIV-positive individuals on clinical trials. Bethesda, MD, National Cancer Institute, 2006.
- Antinori A, Cingolani A, Alba L, et al. Better response to chemotherapy and prolonged survival in AIDS-related lymphomas responding to highly active antiretroviral therapy. AIDS 2001;15:1483-91. [PubMed]
- Sparano JA, Lee S, Chen MG, et al. Phase II trial of infusional cyclophosphamide, doxorubicin, and etoposide in patients with HIV-associated non-Hodgkin's lymphoma: An Eastern Cooperative Oncology Group trial (E1494). J Clin Oncol 2004;22:1491-500. [PubMed]
- Coghill AE, Shiels MS, Suneja G, et al. Elevated Cancer-Specific Mortality among HIV-infected patients in the United States. J Clin Oncol 2015;33:2376-83. [PubMed]
- Vandenhende MA, Roussillon C, Henard S, et al. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. Plos One 2015;10:e0129550-12. [PubMed]
- Velu V, Shetty RD, Larsson M, et al. Role of PD-1 co-inhibitory pathways in HIV infection and potential therapeutic options. Retrovirology 2015;12:14. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 2015;10:e0130142. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004-12. [PubMed]
- Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr LBA109.