MET inhibitors in combination with other therapies in non-small cell lung cancer
Abstract: MET and its ligand hepatocyte growth factor/scatter factor (HGF) influence cell motility and lead to tumor growth, invasion, and angiogenesis. Alterations in MET have been observed in non-small cell lung cancer (NSCLC) tumors, with increased expression associated with more aggressive cancer, as well as acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI). MET inhibitors act via two basic mechanisms. Small molecule inhibitors antagonize ATP in the intracellular tyrosine kinase domain of MET, with studies on the following agents reviewed here: tivantinib (ARQ-197), cabozantinib (XL-184), crizotinib (PF-02341066), amuvatinib (MP470), MGCD265, foretinib (EXEL-2880), MK2461, SGX523, PHA665752, JNJ-38877605, SU11274, and K252A. The monoclonal monovalent antibody fragment onartuzumab (MetMAb) is also discussed here, which binds to and prevents the extracellular activation of the receptor by ligand. MET inhibition may both overcome the negative prognostic effect of MET tumor expression as well as antagonize MET-dependent acquired resistance to EGFR inhibitors. Here we discuss MET inhibitors in combination with other therapies in lung cancer.
Key words: Non-small cell lung cancer; targeted therapies; tyrosine kinase inhibitors; MET
Introduction and impact of the MET signaling axis
Lung cancer remains the leading cause of cancer-related deaths in the United States, estimated to be responsible for 160,000 deaths in 2012, and worldwide killing of more than 1.3 million people annually (1,2). MET is a transmembrane receptor tyrosine kinase (RTK) that binds with high affinity to the ligand hepatocyte growth factor/scatter factor (HGF) (1), and is instrumental in growth of normal epithelial tissues like liver, gastrointestinal tract and kidney (4). MET was first discovered to be linked to cancer in papillary renal cell carcinoma (5), and its function has since been found altered in a variety of cancers including lung cancer (6,7). Upregulation of MET enhances autocrine signaling with HGF and downstream activation of the central proliferation and anti-apoptotic signaling pathways, Ras/Raf/ERK/MAP kinase and PI3 kinase/AKT (8,9); and results in motility, growth, angiogenesis, and invasion in cancers (4,9). Somatic intron mutations in MET create an alternate splicing transcript which deletes the juxtamembrane domain of MET, creating an activated protein with enhanced downstream signaling (10). As previously reviewed, MET is dysregulated via a variety of mechanisms in cancer including MET chromosomal rearrangements, somatic and germline mutations, gene amplification, transcriptional upregulation, and autocrine stimulation via HGF (11).
Increased MET signaling in non-small cell lung cancer (NSCLC) is linked to a relatively unfavorable prognosis. In a study that looked at circulating MET in NSCLC, higher levels were associated with higher nodal stage (P=0.011) and earlier recurrence than lower levels [HR 3.9, 95% confidence interval (CI), 1.17-13.33, P=0.027] (12). In another retrospective study, increased MET gene copy number (GCN) of ≥5 copies/cell were found in 11% NSCLC patients and negatively affected survival (25.8 months in MET positive versus 47.5 months in MET negative tumors, P=0.005) (13). MET also participates in other signaling networks that are important in lung cancer. For example, the epidermal growth factor receptor (EGFR) pathway acts synergistically with MET to increase phosphorylation and activation of downstream effectors, likely through the tyrosine kinase partner ERBB3 (14). Inhibition of both MET and EGFR in vitro decreases cell proliferation by more than double (65.2% versus 21.5-25.5%) than either pathway alone (15). MET amplification or increased HGF expression is associated with 5-50% of acquired resistance to EGFR tyrosine kinase inhibitors (TKIs), like gefitinib or erlotinib (16,17). Theoretically the combination of a MET TKI plus an EGFR TKI may overcome this resistance (14,18-20). Alteration of the MET pathway has also been identified in small cell lung cancer, though the clinical significance is not yet understood (21,22). Based on MET’s importance in cancer development and cross talk between pathways for which there are targeted therapeutics in development, here we review clinical studies involving MET inhibitors in combination with other therapies in the treatment of lung cancer.
Tivantinib (ARQ 197; ArQule, Woburn, Massachusetts)
Tivantinib mechanism of action
Tivantinib is an oral small molecule TKI that binds to the inactive form of MET (23-26). It has shown antitumor activity in xenograft models of colon, gastric, and breast cancer (23). Tivantinib has been studied alone and in combination with other targeted therapeutics, like erlotinib and sorafenib, in a variety of solid tumors.
Tivantinib monotherapy
Five phase I trials were conducted with tivantinib (27). Tivantinib demonstrated linear pharmacokinetics (PK) and inter-patient variable metabolism due to the genetic polymorphism of cytochrome P450 CYP2C19 (28,29). ARQ-101 included 74 patients with metastatic solid tumors (4 with NSCLC), and recommended a phase II dose of 360 mg orally twice daily (bid) of the crystalline formulation (30-32). 3 patients had a partial response (PR) and 40 had stable disease (SD), including two patients with NSCLC, for up to 20 weeks. Because maximum tolerated dose (MTD) was not reached, ARQ-103 (n=51 patients, 1 with NSCLC) was conducted and recommended the phase II dose of 300 mg bid of amorphous formulation (25), which is equivalent to the previously recommended dose of the crystalline formulation (unpublished data, clinicaltrials.gov NCT00658554). Best tumor response was stable disease in 14 patients for ≥4 months. Correlative studies demonstrated a decrease in phosphorylated MET and total MET in 15 available tumor biopsies; decrease in circulating tumor cells in 58% (n=25) of samples; and no significant change in dynamic contrast enhanced magnetic resonance imaging. In these studies, tivantinib was well tolerated with toxicities of greater than 5% including fatigue and nausea, and dose limiting toxicities (DLT) of hematologic cytopenias including febrile neutropenia, fatigue, vomiting, and dehydration (25,32).
Tivantinib combination therapy
Phase I study tivantinib and erlotinib
A phase I study (ARQ 197-111) combined tivantinib in 3 dose cohorts up to 360 mg bid with the standard dose erlotinib (EGFR TKI) 150 mg oral daily (33). All patients had CYP2C19 genotyping performed and intrapatient dose escalation was allowed. Tissue was not required for the study, but when available was tested for mutations in EGFR and KRAS by polymerase chain reaction (PCR), and MET and EGFR amplification by fluorescent in situ hybridization (FISH). Thirty-two patients (mean age =60 years) enrolled had received a median of 3 previous chemotherapies (range, 1-8 chemotherapies), and the most common tumor type was NSCLC (n=8). Six out of eight NSCLC patients had stable disease (3-23 months), of which some had prior exposure to erlotinib. One out of 5 NSCLC patients had an EGFR mutation and 3 out of 6 had MET amplification. Patients with increased MET GCN (3.5-4.5 copies per cell) stayed on study longer than those who did not (14.7 versus 8.6 months). Median progression free survival (PFS) was 4.1 months (95% CI, 2.0-8.1 months). Adverse events (AE) were frequent (87.5%) and included those not seen when tivantinib was used as single agent including grade 1-2 rash and bradycardia. However, previously reported gastrointestinal (GI) symptoms were prevalent along with hematologic toxicities at higher doses (>360 mg bid). The pharmacokinetics of tivantinib were not linear and there were no obvious drug interactions. The recommended phase II dose was tivantinib 360 mg oral bid and erlotinib 150 mg oral daily, although the MTD was not reached.
Phase II study tivantinib and erlotinib
In a randomized double blind phase II study, erlotinib 150 mg oral daily combined with tivantinib 360 mg oral bid (ET) was compared to erlotinib 150 mg oral daily plus placebo (EP) in pre-treated stage IIIB or IV NSCLC patients (34). Patients could not have been exposed to EGFR TKIs, a difference from the phase I trial, and crossover was allowed at progression. The primary end point was PFS and the study was powered to detect a 1.5 month improvement in the ET arm (35). Eighty-four patients were treated with ET and 83 patients with EP. The median age was 63 years and majority of patients were Caucasian, male, current or former smokers, had adenocarcinoma histology (60%), and had received one prior chemotherapy. As KRAS and EGFR status were not used at randomization, there were approximately double the number of patients with tumors harboring a KRAS mutation (10 versus 5) and half the number of patients with tumors harboring EGFR mutations (6 versus 11) in the tivantinib arm, which favored the placebo arm. The majority of patients (73 versus 37) were MET GCN negative as determined by FISH. At a median follow up of 11 months, the trial did not meets it primary endpoint; however, median PFS favored the ET arm (3.8 months versus 2.3 months; HR 0.81, 95% CI, 0.57-1.16, P=0.24). The pre-planned PFS hazard ratio (HR) adjusted for clinical and molecular variables favored the ET arm with PFS HR 0.68 (95% CI, 0.47-0.98, P=0.04). Median overall survival (OS) was not statistically significant different (8.5 months ET versus 6.9 months EP; HR 0.87, 95% CI, 0.59-1.27, P=0.47). Significant benefit was seen with tivantinib in the non-squamous group with adjusted PFS HR 0.61 (95% CI, 0.39-0.98, P=0.04) and OS HR 0.58 (95% CI, 0.34-0.99, P=0.04). In the intention to treat (ITT) population on tivantinib, there was an increase in median time to new metastases and the benefit was again, more evident in the non-squamous group (11 months ET versus 3.6 months EP, HR 0.46, 95% CI, 0.26-0.82, P<0.01). Patients with KRAS mutation tumors had a statistically significant improvement in PFS in the ET arm (HR 0.18, 95% CI, 0.05-0.70, P=0.013, interaction P=0.06), and there was also a trend for improvement in the patients with EGFR wild type tumors (HR 0.70, 95% CI, 0.44-1.10, P=0.12) and higher MET GCN. This regimen may serve as a niche in patients with tumors with KRAS mutations and increased resistance to EGFR TKIs (36). There is an ongoing trial of this combination versus single agent chemotherapy in pretreated advanced NSCLC with KRAS mutations [NCT01395758]. The combination is well tolerated with the most frequent toxicities including rash and diarrhea and both arms had similar rates of grade 3-5 toxicities including anemia and neutropenia.
Phase III study tivantinib and erlotinib
The phase III randomized trial (MARQUEE) studied this combination in stage IIIB/IV non-squamous NSCLC patients with 1 or 2 prior chemotherapies and no prior therapy with an EGFR or MET inhibitor (37). Unlike the phase II trial, patients were randomized by EGFR and KRAS mutation status. They were also tested for but not stratified by tumor MET expression by IHC, MET GCN by FISH, and circulating hepatocyte growth factor (HGF). The trial was terminated in October 2012 after interim analysis showed it would not meet its primary endpoint of overall survival in the approximately 1,000 patients enrolled (http://investors.arqule.com/releasedetail.cfm?ReleaseID=710618). The full results including subset analyses and correlative studies are awaited. The Phase III ATTENTION study of this combination is still ongoing in Asia [NCT01377376].
Cabozantinib (XL-184; Exelixis, South San Francisco, CA)
Cabozantinib mechanism of action
Cabozantinib is a small molecule TKI of MET which also has activity against the VEGFR2, RET, AXL, KIT, FLT-3 and TIE-2 kinases, which are important mediators of tumor cell growth and angiogenesis. It is orally bioavailable and administered once daily. Cabozantinib has been tested in a variety of preclinical and xenograft models, and exerts antitumor effects in mice injected with cells expressing MET and VEGF (38), as well as mouse models of pancreatic neuroendocrine cancer and prostate cancer (39,40). Preclinical efficacy appears to be associated with the inhibitory effects against both MET and VEGFR.
Cabozantinib monotherapy
Multiple phase I studies have been conducted with cabozantinib. In solid tumors, the XL184-001 study treated 85 patients with escalating doses of cabozantinib, with a MTD of 175 mg orally daily (by salt weight, which equals 138 mg freebase equivalent weight) (41). Of note, 35 patients were included with RET-oncogene dependent medullary thyroid cancer, of which 29% achieved partial responses (PR) and an additional 49% had some degree of tumor shrinkage. Major observed toxicities included hand/foot syndrome, diarrhea, and increased risk of hemorrhage.
To investigate the clinical utility of cabozantinib as monotherapy, the XL184-203 phase II trial included a NSCLC cohort as well as 8 other solid tumor cohorts and utilized a randomized discontinuation design (42). Patients received cabozantinib 100 mg (freebase equivalent weight) orally daily for 12 weeks of lead-in, then patients with responses continued cabozantinib, while those with stable disease were randomized to continue either cabozantinib or switch to placebo. The 60 patients with NSCLC received a median of 3 prior lines of therapy, including 50% who had received erlotinib. The radiographic response rate was 10%, with 50% of patients having some degree of objective tumor regression, and the median PFS was 4.2 months. Patients with some degree of tumor regression included, but were not limited to, patients with tumors harboring EGFR and KRAS mutations. Interestingly, the best three responses were observed in patients of untested mutation status, and there was no clear association between histology (adenocarcinoma vs. squamous cell carcinoma) and response. Toxicities included fatigue, diarrhea, anorexia, nausea/vomiting, dysphonia, hypertension, and hand-foot syndrome, with one grade 5 hemorrhage reported in the lead-in stage. The disease stabilization interval among the crossover patients has not yet been reported. An interesting retrospective analysis would be to determine whether any of the best responding tumors harbored RET translocations, which have recently been reported in 1% of NSCLC (43-45).
Cabozantinib combination therapy
In NSCLC, the XL184-202 phase Ib/II study was conducted with the combination of cabozantinib with erlotinib in patients who had developed acquired resistance to erlotinib. In the phase I portion, 64 participants received escalating doses of the combination of erlotinib and cabozantinib (46). Dose limiting toxicities included diarrhea, mucositis, hand-foot syndrome, hypertension, fatigue, hypokalemia, and elevated lipase. Overall, grade 3 or higher events included 43% of patients with diarrhea, 20% with fatigue, 13% with dyspnea, and one grade 5 pulmonary hemorrhage. The MTDs of erlotinib and cabozantinib were 50 mg erlotinib with 125 mg cabozantinib (98 mg freebase equivalent), and 150 mg erlotinib with 50 mg cabozantinib (40 mg freebase equivalent). The response rate was 15%, with a confirmed partial response rate of 8%. Approximately one-third of patients had stable disease for >4 months, lasting up to 15 months. Molecular analysis of tumors revealed no clear association between known EGFR mutations, secondary T790M mutations, and response; but one patient with an EGFR mutation and MET copy number gain by FISH testing did have a partial response. In the phase II expansion, patients were enrolled who failed erlotinib treatment after previous clinical benefit, with the goal of overcoming MET-dependent acquired resistance by the addition of cabozantinib. Patients were randomized to either cabozantinib or cabozantinib plus erlotinib, and the final report of this trial is awaited.
Ongoing studies with cabozantinib in lung cancer include monotherapy with 60 mg (freebase equivalent) in patients with RET translocations [NCT01639508], as well as a three arm randomized study in EGFR wild-type patients to erlotinib (150 mg) versus cabozantinib (60 mg freebase equivalent) versus the combination (150 mg erlotinib and 40 mg cabozantinib) [NCT01708954].
MetMAb (Onartuzumab; Roche, Pleasanton, CA)
Onartuzumab mechanism of action and monotherapy
Onartuzumab is a monovalent one armed 5D5 (OA-5D5) monoclonal antibody (mAb) against MET that blocks binding of HGF as demonstrated in glioblastoma and orthotopic pancreatic tumor models (47,48). Onartuzumab has been studied alone and in combination with bevacizumab in a phase I trial and was well tolerated with adverse events of fatigue, peripheral edema, and hypoalbuminemia (49). Pharmacokinetic studies demonstrated linear pharmacokinetics of onartuzumab from 4 mg/kg to 30 mg/kg (mean clearance values 6.8-9.9 mL/day/kg), an elimination half-life of 8-12 days, and the recommended phase II dose of 15 mg/kg intravenous (iv) every 3 weeks (50,51).
Onartuzumab combination therapy
In a phase II double blind randomized trial (OAM4558g), onartuzumab (15 mg/kg iv every 3 weeks) was studied in combination with erlotinib 150 mg oral daily (OE) versus placebo plus erlotinib (PE) in the second or third line treatment of NSCLC patients who had no previous exposure to an EGFR-TKI (52,53). Tissue was required for all patients enrolled, of which 95% were evaluable for MET IHC and 75% for MET FISH testing. Patients were divided into subgroups based on IHC MET expression, and the immunohistochemical “H-score” of ≥50% of tumor cells staining 2+ or 3+ intensity was defined as MET positive (MET+). Primary endpoints included PFS in both the MET+ population and ITT population. Secondary endpoints included OS and adverse events. In the setting of erlotinib, the majority of patients reached the goal steady state trough concentration of onartuzumab (mean value 73 µg/mL) and there were no drug interactions (51).
There were 128 patients studied, including 65 MET+ patients who had a worse overall survival compared to MET negative (MET -) patients on the placebo plus erlotinib arm (HR 2.52) (53). At a median follow up of 9.9 months, the MET+ group, specifically MET+ by FISH or MET+ by IHC (even if negative by FISH), and irrespective of EGFR status, had an improvement in both PFS and OS when given the combination of onartuzumab plus erlotinib. In the MET+ IHC group, onartuzumab improved time to progression by 2-fold [3 months (OE) versus 1.5 months (PE); HR 0.47, 95% CI, 0.26-0.85, P=0.01] and OS by 3-fold [12.6 months (OE) versus 4.6 months (PE); HR 0.37, 95% CI, 0.2- 0.71, P=0.002]. In a surrogate biomarker analysis that did not specify what chemotherapy was received, there were 12% (13/112 evaluable specimens) EGFR mutations, and these were present in 6 of 7 patients with objective responses (54). MET GCN median copies per cell was 3.44 (range, 1.6-25.0) in 96 evaluable specimens with only 8% gene amplification. KRAS mutations (n=26 specimens) did not affect OS in the onartuzumab arm. The adverse event of peripheral edema was higher in patients who received onartuzumab but otherwise toxicities were well balanced between the two arms (52). MET+ by IHC had the highest sensitivity in detecting clinical improvement with onartuzumab, and establish that diagnostics need to evolve concurrently to identify the appropriate population for targeted therapies. For example, when MET-FISH positivity is defined as ≥5 copies per cell, there was trend to an improved OS (HR 0.47, P=0.19) but not at lower copy numbers (53); and when reverse transcriptase-PCR was used, the OS benefit in MET+ patients was not present at all (HR 0.69, P=0.48) (54).
A phase III double blind randomized study, MetLUNG, is studying the combination of onartuzumab and erlotinib in MET+ (IHC) NSCLC patients who failed one to two chemotherapies (including one platinum based) (55). Patients (N=480) were randomized 1:1 to erlotinib at 150 mg orally daily with either placebo or onartuzumab 15 mg/kg iv every 3 weeks. Although all patients were positive for MET by IHC, they were stratified by degree of MET expression (2+ versus 3+) and also by prior lines of therapy, histology (nonsquamous versus squamous), and presence or absence of EGFR mutations. This trial is ongoing with the primary endpoint of OS and secondary endpoints of PFS, response rate, safety, and PK data [NCT01456325]. Additionally there are ongoing trials of onartuzumab in combination with first line chemotherapy, including bevacizumab and platinum based chemotherapy, in patients with squamous and non-squamous NSCLC [NCT01456325, NCT00854308, NCT01519804, NCT01496742]. These trials will enroll patients regardless of MET IHC with an early safety analysis focused on those with low MET IHC scores.
Crizotinib (PF-02341066; Pfizer, New York, NY)
Crizotinib mechanism of action
Crizotinib was initially characterized as a TKI of MET before it was approved for the treatment of anaplastic lymphoma kinase (ALK) positive NSCLC (56-59). Crizotinib has most activity in MET amplified tumor lines compared to other MET alterations (60). Crizotinib acts synergistically with erlotinib in MET driven NSCLC patient derived xenografts without an ALK mutation to produce complete responses (61).
Crizotinib monotherapy
In a phase I trial, oral crizotinib 250 mg twice daily was given to ALK-positive stage III or IV NSCLC (58,59), of which majority were previously treated nonsmokers with adenocarcinoma histology (97%). Clinical activity was noted with significant durable responses: 60.8% overall response rate (ORR) (95% CI, 52.3-68.9) with 3 complete responses (CR) and 84 partial responses (PR); median duration of response (DOR) of 49.1 weeks (95% CI, 39.3-75.4 weeks); and median PFS of 9.7 months (95% CI, 7.7-12.8 months). Crizotinib was well tolerated with the most common adverse events of GI and visual disturbance (>40%), and peripheral edema (30%). Grade 3-4 events included neutropenia/lymphopenia (n=15) and elevated ALT (n=6). Tumors available from 33 patients were tested and were negative for MET amplification, suggesting that MET was not responsible for the crizotinib treatment responses (58). However, the MET alteration of MET gene amplification is relatively uncommon. In a phase II trial (PROFILE 1005), crizotinib was evaluated in at least the second line setting for advanced ALK positive NSCLC (62) with similar demographics and results to the phase I study [ORR of 53%, median DOR 43 weeks (95% CI, 36-50 weeks), median PFS of 8.5 months (95% CI, 6.2-9.9 months)]. 29 patients had treatment related SAE including dyspnea and pneumonitis and febrile neutropenia. The phase III study demonstrated superiority of crizotinib to standard chemotherapy of either pemetrexed or docetaxel in previously treated ALK positive advanced lung cancer. Patients (n=374) showed an improvement in median PFS of 7.7 versus 3 months (HR 0.49; 95% CI, 0.37-0.64, P<0.0001), and an ORR of 65% versus 20% (P<0.0001) (63).
Crizotinib combination therapy
A phase I/II study is ongoing with the combination of crizotinib 150 mg bid or 200 mg bid with erlotinib 100 mg daily in patients with pre-treated NSCLC and no prior MET targeted therapy (64). Twenty-five patients have enrolled and median duration of therapy is similar at the lower combination dose (6.6 weeks, n=18) and the higher combination dose (6.9 weeks, n=7). Five patients had DLT’s (all resolved) at both dose combinations with predominance of GI events: grade 2 vomiting, grade 2 esophagitis and dysphagia, grade 3 diarrhea and dehydration, grade 3 esophagitis, and grade 3 dry eye. The most common treatment related AE’s included grade 1-2 diarrhea (72%), rash (56%), and fatigue (44%); and 6 patients had to discontinue therapy. There was one partial response at the higher dose of crizotinib of 61 weeks duration and 9 with stable disease (duration 7-54 weeks). Pharmacokinetics demonstrated a drug interaction with increase in erlotinib AUC by 1.8 fold. The MTD was reached of erlotinib 100 mg daily and crizotinib 150 mg bid [NCT00965731]. As seen in Tables 1 and 2, there are a variety of crizotinib combination trials that are recruiting with pan-Her inhibitor PF-0299804 [NCT01441128], ganetespib (STA 9090) [NCT01579994], and pazopanib [NCT01548144].
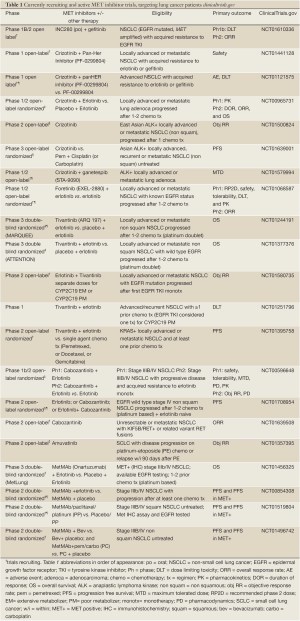
Full table
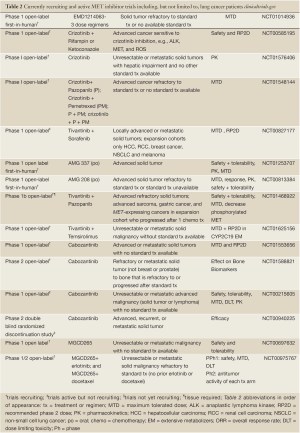
Full table
MET inhibitors in clinical development
Amuvatinib (MP470; Astex, Pleasanton, CA)
Amuvatinib (MP470) was developed for activity against imatinib resistant gastrointestinal stromal tumors (GIST), in which cKIT is downregulated, and tyrosine kinase receptors, AXL and MET are upregulated (65). Amuvatinib is a multi-targeted inhibitor of MET, AXL, and platelet derived growth factor receptor alpha (PDGFRα). It also acts as a radiosensitizer via inhibition of homologous recombination protein RAD51 (66,67), a known poor prognostic marker in NSCLC with decreased median survival time of 19 versus 68 months (68). Amuvatinib plus erlotinib have shown synergy with enhanced downstream AKT inhibition and tumor suppression in prostate cancer xenograft mice (69).
In early phase I cancer studies, amuvatinib dry powder capsules (DPC) did show activity but low systemic exposure, and a new lipid suspension formulation (LSC) was tested (70). In three phase I studies (n=58 healthy subjects), the new LSC suspension, multiple dosing regimens, and high fat meals increased drug exposure. A phase Ib study of a standard 3+3 design, enrolled cancer patients to receive amuvatinib (DPC) uninterrupted in combination with five standard of care chemotherapy regimens (21 day cycles) (71,72). Amuvatinib doses were escalated from 100 mg oral daily to 400 mg oral twice daily until the MTD of the drug with each regimen was reached. A total of 100 patients (mean age =58) were enrolled, of which 90% were pretreated. The standard of care chemotherapy regimens included: paclitaxel and carboplatin (n=23), carboplatin and etoposide (n=22), topotecan (n=15), docetaxel (n=15), and erlotinib (n=20). There were no drug interactions except with the docetaxel arm and the MTD was not reached. The most common treatment related AEs were expected of each chemotherapy regimen. Clinical activity was noted with 12 partial responses, with majority in the platinum based chemotherapy arms (n=11), and 5 of 11 small cell lung cancer and neuroendocrine tumor patients (72) and 1 NSCLC patient (71). Forty four patients had stable disease, for an overall disease control rate of 56% (95% CI, 46-66). Pharmacodynamic studies confirmed Rad51 inhibition and increased DNA damage on skin biopsies. Amuvatinib may serve a niche in small cell lung cancer, and a study is ongoing in those who have progressed on platinum and etoposide [NCT01357395].
MGCD265 (Methylgene, Montreal, Canada)
MGCD265 is an oral TKI of MET, VEGFR (1-3), Tie and Ron (73). Non-small cell lung cancer xenograft models including one that harbored the TKI resistant EGFR mutation T790M, combined MGCD265 with either docetaxel, paclitaxel, or erlotinib. Each combination elicited greater tumor response than either agent alone, and displayed antiangiogenic properties with docetaxel (74).
In a phase I study, MGCD265 was given orally from 24 mg/m2 daily to 235 mg/m2 twice daily uninterrupted to patients with advanced solid malignancy until disease progression (75,76). As of January 2012, a total of 56 patients (median age =61 years) were enrolled (76). Six patients had stable disease for ≥6 cycles (range, 6-12 cycles). Dose limiting toxicities occurred at daily doses ≥250 mg/m2 and included grade 2 hypertension and grade 3 elevated lipase, fatigue, and pituitary hemorrhage. Pharmacokinetics were linear and the half-life was long (26 + hours) (77), and twice daily compared to daily dosing increased exposure along with MET and VEGFR inhibition (76). Other variables including intermittent dosing schedules and a capsule formulation have been tried and demonstrate efficacy and are well tolerated (77-79).
MGCD265 has been studied in a variety of advanced solid tumors including NSCLC, as a monotherapy (75-79) and in combination with either docetaxel (80,81) or erlotinib (73,82). In a phase I standard 3+3 dose escalation, MGCD265 (96 mg/m2 once daily up to 162 mg/m2 bid) was combined with erlotinib at 100 or 150 mg daily to determine safety (73,82), and 45 patients have been enrolled (73,82). Dose limiting toxicity was present at the lowest dose combination and included diarrhea (n=1), and at the higher dose cohorts, rash and fatigue (n=1) and rhabdomyolysis (n=1). One of three NSCLC patients with an EGFR mutation had a partial response for 8 cycles, and 7 patients had stable disease for ≥6 cycles. Pharmacodynamic studies showed a decrease in HGF (MET ligand) plasma levels at day 8 of cycle one.
In a separate phase I dose escalating trial, MGCD265 was combined with docetaxel (50 then 75 mg/m2 iv once every 3 weeks) in advanced solid tumors (n=34), including 9 NSCLC patients (80,81). The MTD of the microionized formulation of MGCD265 is 72 mg/m2 bid and docetaxel 75 mg/m2 every 3 weeks but has not been reached with the new formulation (80). NSCLC contributed to half (n=2) of the partial responses seen that lasted up to 8 cycles (81), and half (n=3) of stable disease seen that lasted ≥6 cycles (up to 18 cycles) (80). Four out of the 5 patients with NSCLC with PR or SD had received prior taxane therapy and 3 of their available tumor samples had high levels of MET and phosphorylated-MET expression, suggesting synergy with the combination of taxanes and MGCD265 (80). Pharmacodynamic studies also showed decreased HGF levels at day 8 compared to baseline. The combination was well tolerated with grade 3 toxicities including diarrhea, elevated lipase, neutropenia, and leukopenia (80,81). Based on MGCD265 activity in NSCLC and potential synergistic effect with taxanes, a trial is currently recruiting for MGCD265 with either docetaxel or erlotinib in taxane or erlotinib naive pre-treated advanced NSCLC patients [NCT00975767].
MET inhibitors: SGX523, Foretinib (EXEL-2880, XL 880, GSK1363089), MK2461, and others
SGX523 is a MET TKI that binds to its inactive form (83,84) and has shown activity in MET amplified lung cancer cell lines (83). The combination of erlotinib and SGX523 inhibited growth in a NSCLC tumor xenograft by almost 3-fold over each agent alone (85). Unfortunately, a phase I open label dose escalation study of orally administered SGX523 on a twice daily continuous schedule in advanced cancer had to be terminated because of unexpected metabolite induced renal toxicity [(86), NCT00606879].
Foretinib is an oral multitargeted TKI of primarily MET and VEGF-2 along with Kit, FLT3, platelet derived growth factorβ, and Tie2 (87). Foretinib has been studied in phase I/II trials in a variety of advanced solid tumors including mesothelioma (88). Foretinib combined with erlotinib increases sensitivity in MET and EGFR amplified lung tumor cell lines in the presence of HGF (MET ligand) (89). The first phase I study in advanced solid tumors reached a MTD of 3.6 mg/kg with a recommended dose of 240 mg for 5 days of a 14 day cycle (90) with DLT’s including grade 3 transaminases and elevated lipase. The combination of foretinib and erlotinib is being compared to erlotinib in pretreated advanced NSCLC with correlative studies requiring archival tumor and EGFR status [NCT01068587].
MK-2461 is an oral multitargeted TKI of activated MET, fibroblast growth factor receptor (FGFR), and platelet-derived growth factor amongst others (91,92). Despite more potent binding to MET in vitro, there was more anti-proliferative activity in FGFR-2 driven cell lines (91). MK-2461 daily or bid has been studied in a phase I dose escalation trial in refractory advanced solid tumors (93). Patients (n=14) received 1-6 cycles that were well tolerated, and no tumor responses were seen. There are no trials of MK-2461 in lung cancer. Other MET inhibitors in development that show activity preclinically in lung cancer, include PHA66572 (94-99), JNJ-38877605 (100), SU11274 (101,102), and K252A (103-104).
Conclusions
MET inhibitors may add to the lung cancer treatment landscape and can enhance cell death and tumor growth when combined with chemotherapy and radiation (106). As reviewed in this paper, combination therapy with MET inhibition is under development to both increase the efficacy of primary therapies and to overcome resistance to targeted agents like erlotinib in lung cancer (14,18,19). With all targeted therapies, including MET inhibitors, resistance eventually develops. Mechanisms include mutation in MET structure and increased expression of TGF alpha/EGFR, both resulting in downstream activation of PI3K-AKT and MEK/MAP/ERK kinase pathways (107, 108), along with KRAS gene amplification (109). Eventually resistance also develops to combination therapies targeting MET and EGFR pathways, and STAT3 and BCL-2 signaling have been implicated (110).
The three MET inhibitors furthest in development are tivantinib, cabozantinib, and onartuzumab. For example, despite setbacks for tivantinib with the negative phase III lung cancer MARQUEE study, there is still potential for benefit in the KRAS mutant population and in other subsets distinguished by specific biomarkers (34). Cabozantinib has also demonstrated efficacy in heavily pretreated NSCLC patients (including those with squamous histology) and also in combination with erlotinib in patients who had previously received an EGFR TKI (42,46). Onartuzumab has a unique MET inhibition mechanism as a monovalent one armed 5D5 monoclonal antibody and has shown significant improvement in PFS and OS in MET IHC positive tumors when combined with erlotinib (52,53). Other particularly promising MET inhibitors include crizotinib and amuvatinib, of which the latter is being tested in small cell lung cancer where the role of MET is less clear (72) [NCT01357395]. As mentioned in Tables 1,2, several trials are ongoing looking at MET inhibitors as monotherapy and in combination therapy in lung cancer. Other inhibitors of the MET axis are being studied, such as the HGF ligand inhibitor AMG102, in combination with platinum based chemotherapy in extensive stage small cell lung cancer [NCT00791154] and in combination with erlotinib in NSCLC [NCT01233687]. Inhibitors of the MET/HGF signaling pathway shows great promise in the treatment of lung cancer in combination with chemotherapy and with EGFR targeted agents such as erlotinib. Their true potential has yet to be reached and will hopefully be realized with better understanding of biomarkers allowing for proper patient selection.
Acknowledgements
Disclosure: The content of this paper has not been published and is not being submitted elsewhere for publication. Dr. Heather Wakelee has ongoing clinical trials with Pfizer, Exelixis, and Genentech and receives research funding to conduct the trials. Dr. Joel Neal has ongoing clinical trials with Genentech and ArQule and receives research funding to conduct the trials. Dr. Sukhmani Padda has no disclosures.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
- Naldini L, Weidner KM, Vigna E, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 1991;10:2867-78.
- Di Renzo MF, Narsimhan RP, Olivero M, et al. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 1991;6:1997-2003.
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73.
- Tsao MS, Liu N, Chen JR, et al. Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer 1998;20:1-16.
- Olivero M, Rizzo M, Madeddu R, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer 1996;74:1862-8.
- Wright TG, Singh VK, Li JJ, et al. Increased production and secretion of HGF alpha-chain and an antagonistic HGF fragment in a human breast cancer progression model. Int J Cancer 2009;125:1004-15.
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915-25.
- Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res 2006;66:283-9.
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16.
- Cheng TL, Chang MY, Huang SY, et al. Overexpression of circulating c-met messenger RNA is significantly correlated with nodal stage and early recurrence in non-small cell lung cancer. Chest 2005;128:1453-60.
- Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 2009;27:1667-74.
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43.
- Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog 2008;7:9.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26.
- Yano S, Yamada T, Takeuchi S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol 2011;6:2011-7.
- Cappuzzo F, Janne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009;20:298-304.
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7.
- Xu L, Kikuchi E, Xu C, et al. Combined EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of lung cancers codriven by mutant EGFR containing T790M and MET. Cancer Res 2012;72:3302-11.
- Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003;63:6272-81.
- Maulik G, Kijima T, Ma PC, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res 2002;8:620-7.
- Munshi N, Jeay S, Li Y, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther 2010;9:1544-53.
- Eathiraj S, Palma R, Volckova E, et al. Discovery of a novel mode of protein kinase inhibition characterized by the mechanism of inhibition of human mesenchymal-epithelial transition factor (c-Met) protein autophosphorylation by ARQ 197. J Biol Chem 2011;286:20666-76.
- Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol 2011;29:1271-9.
- Abbadessa G, Chai F, Waghorne C, et al. Biomarker results from five phase I studies of ARQ 197, an oral c-MET inhibitor. 2010 Molecular Markers Hollywood, FL. 2010:abstr 85.
- Adjei AA, Schwartz B, Garmey E. Early clinical development of ARQ 197, a selective, non-ATP-competitive inhibitor targeting MET tyrosine kinase for the treatment of advanced cancers. Oncologist 2011;16:788-99.
- Desta Z, Zhao X, Shin JG, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002;41:913-58.
- Nishina T, Hirashima T, Sugio K, et al. The effect of CYP2C19 polymorphism on the tolerability of ARQ 197: Results from phase I trial in Japanese patients with metastatic solid tumors. J Clin Oncol 2011;29:abstr 2516.
- Garcia A, Rosen L, Cunningham CC, et al. Phase 1 study of ARQ 197, a selective inhibitor of the c-Met RTK in patients with metastatic solid tumors reaches recommended phase 2 dose. J Clin Oncol 2007;25:abstr 3525.
- Mekhail T, Rich T, Rosen L, et al. Final results: A dose escalation phase I study of ARQ 197, a selective c-Met inhibitor, in patients with metastatic solid tumors. J Clin Oncol 2009;27:abstr 3548.
- Rosen LS, Senzer N, Mekhail T, et al. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res 2011;17:7754-64.
- Goldman JW, Laux I, Chai F, et al. Phase 1 dose-escalation trial evaluating the combination of the selective MET (mesenchymal-epithelial transition factor) inhibitor tivantinib (ARQ 197) plus erlotinib. Cancer 2012. [Epub ahead of print].
- Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 2011;29:3307-15.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32.
- Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res 2007;13:2890-6.
- Scagliotti GV, Novello S, Schiller JH, et al. Rationale and Design of MARQUEE: A Phase III, Randomized, Double-Blind Study of Tivantinib Plus Erlotinib Versus Placebo Plus Erlotinib in Previously Treated Patients With Locally Advanced or Metastatic, Nonsquamous, Non-Small-Cell Lung Cancer. Clin Lung Cancer 2012;13:391-5.
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298-308.
- Zhang S, Zhau HE, Osunkoya AO, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer 2010;9:9.
- Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012;2:270-87.
- Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660-6.
- Hellerstedt BA, Edelman G, Vogelzang NJ, et al. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT). J Clin Oncol 2012;30:abstr 7514.
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4.
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81.
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7.
- Wakelee HA, Gettinger SN, Engelman JA, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:abstr 3017.
- Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res 2008;68:4360-8.
- Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res 2006;12:6144-52.
- Moss RA, Patel P, Bothos J, et al. Complete Results from Phase I Dose Escalation Study of MetMAb, a monovalent antagonist antibody to the receptor MET, dosed as single agent and in combination with bevacizumab in patients with advanced solid malignancies. Ann Oncol 2010;21:abstr 504P.
- Moss RA, Bothos JG, Filvaroff E, et al. Phase Ib dose-escalation study of MetMAb, a monovalent antagonist antibody to the receptor MET, in combination with bevacizumab in patients with locally advanced or metastatic solid tumors. J Clin Oncol 2010;28:abstr e13050.
- Bai S, Xin Y, Jin D, et al. Population pharmacokinetic analysis from phase I and phase II studies of the humanized monovalent antibody, MetMAb, in patients with advanced solid tumors. J Clin Oncol 2011;29:abstr 2571.
- Vashishtha A, Patel PH, Yu W, et al. Safety data and patterns of progression in met diagnostic subgroups in OAM4558g; A phase II trial evaluating MetMAb in combination with erlotinib in advanced NSCLC. J Clin Oncol 2011;29:abstr 7604.
- Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol 2011;29:abstr 7505.
- Yu W, Pandita A, Penuel E, et al. Exploratory biomarker analyses from OAM4558g: A placebo-controlled phase II study of erlotinib with or without MetMAb in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2011;29:abstr 7529.
- Spigel DR, Edelman MJ, Mok T, et al. The MetLUNG study: A randomized, double-blind, phase III study of onartuzumab (MetMAb) plus erlotinib versus placebo plus erlotinib in patients with advanced, MET-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr TPS7616.
- Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007;67:4408-17.
- Cui JJ, Tran-Dube M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011;54:6342-63.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703.
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9.
- Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol 2011;6:1624-31.
- Yang M, Shan B, Li Q, et al. Overcoming erlotinib resistance with tailored treatment regimen in patient-derived xenografts from naive Asian NSCLC patients. Int J Cancer 2012. [Epub ahead of print].
- Kim DW, Ahn M, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J CLin Oncol; 2012;30:abstr 7533.
- Shaw AT, Kim DW, Nakagawa K, et al. PHASE III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK positive non small cell lung cancer (PROFILE 1007). Ann Oncol 2012;23:abstr LBA1 PR.
- Ou SI, Govindan R, Eaton KD, et al. Phase I/II dose-finding study of crizotinib (CRIZ) in combination with erlotinib (E) in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 2610.
- Mahadevan D, Cooke L, Riley C, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 2007;26:3909-19.
- Welsh JW, Mahadevan D, Ellsworth R, et al. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol 2009;4:69.
- Zhao H, Luoto KR, Meng AX, et al. The receptor tyrosine kinase inhibitor amuvatinib (MP470) sensitizes tumor cells to radio- and chemo-therapies in part by inhibiting homologous recombination. Radiother Oncol 2011;101:59-65.
- Qiao GB, Wu YL, Yang XN, et al. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer 2005;93:137-43.
- Qi W, Cooke LS, Stejskal A, et al. MP470, a novel receptor tyrosine kinase inhibitor, in combination with Erlotinib inhibits the HER family/PI3K/Akt pathway and tumor growth in prostate cancer. BMC Cancer 2009;9:142.
- Choy G, Joshi-Hangal R, Oganesian A, et al. Safety, tolerability, and pharmacokinetics of amuvatinib from three phase 1 clinical studies in healthy volunteers. Cancer Chemother Pharmacol 2012;70:183-90.
- Mita MM, Tolcher A, Gordon MS, et al. A phase Ib dose-escalation study of orally administered MP-470, a multi-kinase inhibitor and supressor of Rad51, in combination with carboplatin doublet containing regimens shows activity in highly refractory solid tumor patients. J Clin Oncol 2009;27:abstr e13511.
- Sankhala KK, Tolcher AW, Mita MM, et al. Amuvatinib (MP-470), an oral dual inhibitor of mutant kinases and DNA repair: Final results from a 100-patient, 5-arm phase Ib trial in combination with five standard of care (SOC) anticancer regimens. J Clin Oncol 2011;29:abstr 3074.
- O’Dwyer PJ, Papadopoulos KP, Tolcher AW, et al. MGCD265, a multitargeted oral tyrosine kinase receptor inhibitor of Met and VEGFR, in combination with erlotinib in patients with advanced solid tumors. J Clin Oncol 2012;30:abstr e13602.
- Besterman JM, Fournel M, Dupont I, et al. Potent preclinical antitumor activity of MGCD265, an oral Met/VEGFR kinase inhibitor in phase II clinical development, in combination with taxanes or erlotinib. J Clin Oncol 2010;28:abstr e13595.
- Kollmannsberger CK, Hurwitz H, Vlahovic G, et al. Phase I study of daily administration of MGCD265 to patients with advanced malignancies (Study 265-101). J Clin Oncol 2009;27:abstr e14525.
- Kollmannsberger CK, Hurwitz H, Cleary JM, et al. MGCD265, a multitargeted oral tyrosine kinase receptor inhibitor of Met and VEGFR: Dose-escalation phase I study (abstr 3039). 2012 ASCO Annual Meeting Chicago, IL. J Clin Oncol 2012;30:abstr 3039.
- Drouin MA, Kollmannsberger CK, Uronis HE, et al. Daily administration of MGCD265 to patients with solid tumors in a dose-escalation phase I study (study 265-101). J Clin Oncol 2010;28:abstr 3106.
- Hong D, LoRusso P, Kurzrock R, et al. Phase I study of MGCD265 administered intermittently to patients with advanced malignancies (Study 265-102). J Clin Oncol 2009;27:abstr e14516.
- Heath EI, LoRusso P, Kurzrock R, et al. A phase I study of oral administration of MGCD265 in patients with advanced malignancies (study 265-102). J Clin Oncol 2010;28:abstr 3108.
- Rasco DW, Patnaik A, Amaravadi RK, et al. Determination of the maximum tolerated dose (MTD) of MGCD265, an oral Met/VEGFR multitargeted receptor tyrosine kinase inhibitor, in combination with docetaxel. J Clin Oncol 2011;29:abstr 3032.
- Beeram M, Patnaik A, Amaravadi RK, et al. MGCD265, a multitargeted oral tyrosine kinase receptor inhibitor of Met and VEGFR, in combination with docetaxel. J Clin Oncol 2012;30:abstr e13604.
- O’Dwyer PJ, Papadopoulos KP, Tolcher AW, et al. MGCD265, an oral Met/VEGFR multitargeted receptor tyrosine kinase inhibitor, in combination with erlotinib: Phase I clinical experience. J Clin Oncol 2011;29:abstr 3083.
- Buchanan SG, Hendle J, Lee PS, et al. SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol Cancer Ther 2009;8:3181-90.
- Guessous F, Zhang Y, diPierro C, et al. An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anticancer Agents Med Chem 2010;10:28-35.
- Zhang YW, Staal B, Essenburg C, et al. MET kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor-dependent fashion to suppress carcinoma growth. Cancer Res 2010;70:6880-90.
- Infante JR, Rugg T, Gordon M, et al. Unexpected renal toxicity associated with SGX523, a small molecule inhibitor of MET. Invest New Drugs 2012. [Epub ahead of print].
- Qian F, Engst S, Yamaguchi K, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res 2009;69:8009-16.
- Naing A, Kurzrock R, Adams LM, et al. A comparison of the pharmacokinetics of the anticancer MET inhibitor foretinib free base tablet formulation to bisphosphate salt capsule formulation in patients with solid tumors. Invest New Drugs 2012;30:327-34.
- Liu L, Shi H, Liu Y, et al. Synergistic effects of foretinib with HER-targeted agents in MET and HER1- or HER2-coactivated tumor cells. Mol Cancer Ther 2011;10:518-30.
- Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res 2010;16:3507-16.
- Pan BS, Chan GK, Chenard M, et al. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res 2010;70:1524-33.
- Katz JD, Jewell JP, Guerin DJ, et al. Discovery of a 5H-benzo[4,5]cyclohepta[1,2-b]pyridin-5-one (MK-2461) inhibitor of c-Met kinase for the treatment of cancer. J Med Chem 2011;54:4092-108.
- Camacho LH, Moulder SL, LoRusso PM, et al. First in human phase I study of MK-2461, a small molecule inhibitor of c-Met, for patients with advanced solid tumors. J Clin Oncol 2008;26:abstr 14657.
- Medová M, Aebersold DM, Zimmer Y. MET inhibition in tumor cells by PHA665752 impairs homologous recombination repair of DNA double strand breaks. Int J Cancer 2012;130:728-34.
- Puri N, Khramtsov A, Ahmed S, et al. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res 2007;67:3529-34.
- Arriola E, Canadas I, Arumi-Uria M, et al. MET phosphorylation predicts poor outcome in small cell lung carcinoma and its inhibition blocks HGF-induced effects in MET mutant cell lines. Br J Cancer 2011;105:814-23.
- Mukohara T, Civiello G, Davis IJ, et al. Inhibition of the met receptor in mesothelioma. Clin Cancer Res 2005;11:8122-30.
- Brevet M, Shimizu S, Bott MJ, et al. Coactivation of receptor tyrosine kinases in malignant mesothelioma as a rationale for combination targeted therapy. J Thorac Oncol 2011;6:864-74.
- Yang Y, Wislez M, Fujimoto N, et al. A selective small molecule inhibitor of c-Met, PHA-665752, reverses lung premalignancy induced by mutant K-ras. Mol Cancer Ther 2008;7:952-60.
- King P, Janssens B, Verhulst T, et al. JNJ-38877605: A selective oral Met inhibitor for the treatment of cancer 101st American Association for Cancer Research Annual Meeting; Washington DC. 2010.
- Liu Y, Yang Y, Ye YC, et al. Activation of ERK-p53 and ERK-mediated phosphorylation of Bcl-2 are involved in autophagic cell death induced by the c-Met inhibitor SU11274 in human lung cancer A549 cells. J Pharmacol Sci 2012;118:423-32.
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88.
- Morotti A, Mila S, Accornero P, et al. K252a inhibits the oncogenic properties of Met, the HGF receptor. Oncogene 2002;21:4885-93.
- Schiering N, Knapp S, Marconi M, et al. Crystal structure of the tyrosine kinase domain of the hepatocyte growth factor receptor c-Met and its complex with the microbial alkaloid K-252a. Proc Natl Acad Sci U S A 2003;100:12654-9.
- Perez-Pinera P, Hernandez T, Garcia-Suarez O, et al. The Trk tyrosine kinase inhibitor K252a regulates growth of lung adenocarcinomas. Mol Cell Biochem 2007;295:19-26.
- Medová M, Aebersold DM, Blank-Liss W, et al. MET Inhibition Results in DNA Breaks and Synergistically Sensitizes Tumor Cells to DNA-Damaging Agents Potentially by Breaching a Damage-Induced Checkpoint Arrest. Genes Cancer 2010;1:1053-62.
- Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011;71:1081-91.
- McDermott U, Pusapati RV, Christensen JG, et al. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res 2010;70:1625-34.
- Cepero V, Sierra JR, Corso S, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res 2010;70:7580-90.
- Ma PC, Fan W, Tang Z, et al. Role of antiapoptotic STAT3 and BCL-2 signaling in promoting lung tumor cells to survive against combined MET-/EGFR-targeted inhibitors. J Clin Oncol 2010;28:abstr 10644.


