Pulmonary adenocarcinoma histology
Modified WHO classification of adenocarcinomas
This review will focus on morphologic diagnosis of adenocarcinoma, pointing to areas where improvements for diagnosis are possible, show the prognostic relevance of the different patterns. Reviews on how to handle specimens for predictive analysis of NSCLC have recently been published (1,2).
Interestingly, the WHO classification of lung cancer is based on resection specimen examination, while only about 10-20% of patients with lung cancer are amenable to surgery. In most cases the diagnosis of lung cancer is performed on cytology and small biopsy samples obtained by various means (2). The recent multidisciplinary classification of lung adenocarcinoma extends the classification to such small samples (3). In the morphological classification, primarily of surgically resected tumors, 5 invasive tumor patterns are recognised: adenocarcinoma with lepidic growth pattern, acinar pattern, papillary pattern, micropapillary pattern, and solid pattern with mucin. When the lesion is purely lepidic and 3 cm or less in diameter, adenocarcinoma in situ (AIS) is diagnosed. Such a lesion is considered a non-invasive carcinoma with a 100% five year survival rate.
Distinction of well differentiated adenocarcinomas from well differentiated squamous cell carcinomas is generally good, but less easy when the carcinomas are poorly differentiated. Given that most cases of pulmonary adenocarcinoma show mixed morphology in relation to the five major histological patterns, evidence shows that a predominant pattern can be reproducibly identified with high concordance amongst pathologists in resection specimens (4). However, in this study partly designed to uncover differences in interpretation, recognition of the adenocarcinoma in situ pattern is more problematic, though kappa values are fair. This area may be improved by having more precise definitions and subsequent better education on interpretation of existing terminology, and/or additional markers of invasion. The prime confounder is using a two dimensional histological section to diagnose a lesion with a complex three dimensional architecture, an issue that is not adequately addressed within current definitions. In addition, also other factors contributed to the variation in interpretation: (I) a stromal component was considered as tumour-related stroma with fibroblasts (also called desmoplastic stroma), while others considered the same feature benign scarring/fibroelastosis; (II) the presence of elastin was variably weighed as representing native alveolar wall by some pathologists but not by others; (III) inflammation in alveolar walls implied invasive disease to some; (IV) although there was good agreement between pathologists in cases with a prominent micropapillary component, there was variation in interpretation between what some interpreted as focal micropapillary component and tangential cutting of both lepidic and true papillary structures; (V) some pathologists interpreted a mucinous lepidic component as being invasive, based upon the reasonable assumption that elsewhere in the tumour an invasive component with scarring is highly likely, while others interpreted the image in itself as non-invasive. It is therefore notable that much of the interobserver variation stems from interpretation based on operator experience and opinion and improved definitions and better education on their usage are required to reduce interobserver variability. Although within one educational setting high agreement is feasible (5-7), differences in interpretation do still occur.
Resection artefact and classification of adenocarcinoma
Surgical removal and pathological handing of resected lung tissue may have a compressive effect on the alveolar lung tissue, distorting its architecture. To facilitate a pulmonary resection and improve access in the thoracic cavity, the lung is usually deflated during segmentectomy, lobectomy and pneumonectomy. Especially during video-assisted thoracoscopic surgery (VATS) procedures, visualization of the surgical field is seriously hampered by a ventilated lung. The effect of surgical atelectasis on morphology has not been examined in depth with respect to lung adenocarcinomas.
In histologic sections the deflation effect of surgical atelectasis induces ‘lack of empty space’ equivalent to air in vivo. If the open biopsy or resection specimen is not subsequently perfusion fixed, either through the bronchus, or by injection through the visceral pleura, this artefact remains, as is common in daily practice. This surgical atelectasis is a prime confounder in reproducibility of adenocarcinomas subtypes, as this gives rise to a misleading morphologic appearance of a papillary pattern, while actually a lepidic pattern is present (8). It should be recognised that, in specimens where the alveolar tissues are compressed or deflated, a pseudopapillary pattern may be evident where tumor cells appear to line central fibrovascular cores. These cores lacking a myofibroblastic stroma but showing discontinuous elastic fibres and an obvious lepidic pattern at the edge are clues to the correct diagnosis of AIS. In addition, pigmented alveolar macrophages in acinar lumen point to a re-existing alveolar structure (9).
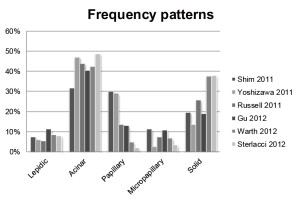
Frequency of patterns and prognosis
Since the multidisciplinary classification of pulmonary adenocarcinoma several studies examined the frequency of occurrence of the patterns and also the relation with prognosis either by 5 years overall survival or by 5 years disease free survival. So far 6 studies described the frequency of occurrence of the different patterns (10-15), see Figure 1. The most frequently diagnosed pattern is the acinar pattern. The second is the solid by some and papillary pattern by others. The biggest range in frequency is noted for papillary, micropapillary and solid pattern. This variation may be explained by differences in interpretation of the patterns.
Grouping the patterns into well differentiated (lepidic), moderately differentiated (acinar and papillar) and poorly differentiated (micropapillar and solid) reveals for these studies a prognostic difference. For the 5 years disease free survival the data of 4 studies are shown in Figure 2 (10,13,15).
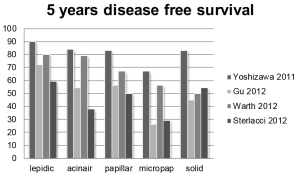
Remarkably for all patterns there is at least a difference in prognosis of 30% between these 4 studies. Moreover, the paper of Yoshisawa et al. shows a high DFS for all patterns being the lowest for (67%) for the micropapillary pattern (15), in contrast to the others. Although there may be several reasons to explain these prognostic differences, one of these may be that a high fraction of cases are called predominant papillary or acinar carcinoma which might be called lepidic by other pathologists, introducing a kind of stage shift with improved prognosis.
Biomarkers for diagnosis of adenocarcinoma
As only 10-20% of lung cancers are amenable for resection, more diagnosis of lung cancer are made on biopsies and cytology specimen. These are frequently obtained in cases with higher stage. In smaller samples, the chance is higher that a well differentiated area is lacking compared to resection specimen, explaining the difficulty of distinguishing adenocarcinoma from squamous cell carcinoma in biopsies (2). This morphological grey zone in biopsies is called non-small cell lung carcinoma nototherwise specified (NSCLC-NOS) and may comprise 20-40% of the biopsy specimen (3). The help of additional stains reduces this number to 4-6%, with NSCLC-NOS ‘favour adenocarcinoma’ or ‘favour squamous cell carcinoma’ as useful terms in daily practice. The stains in this context are TTF1, Napsin A and mucin for adeno-differentiation and p63/p40 for squamous cell carcinoma (16-27). In the application of p63 only a strong staining in >80% of the basally located tumor nuclei points to squamous differentiation, as p63 is negative in better differentiated part of squamous cell carcinoma and sometimes weakly positive in adenocarcinomas (24). Napsin A and TTF1 stain adenocarcinomas, with an approximately similar frequency with slightly better performance for TTF1 in some (28), and Napsin A in other studies (17,27). In diagnosis of NSCLC on small samples liberal use of the category NOS is advocated, with subsequent use of additional stains, as the distinction between adenocarcinomas and squamous cell carcinomas forms in metastatic lung cancer the basis for treatment selection and predictive biomarker testing.
In conclusion, the accurate appreciation of tumor architecture in lung adenocarcinoma has important biological and clinical implications. Interobserver variation may explain differences in frequency of subtype and prognosis. In diagnosis of small samples immunohistochemistry is frequently helpful in the distinction of adenocarcinomas from squamous cell carcinomas.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 2012;461:245-57.
- Thunnissen E, Kerr KM, Herth FJ, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung Cancer 2012;76:1-18.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85.
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012. [Epub ahead of print].
- Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. Am J Surg Pathol 2006;30:606-13.
- Thunnissen FB, Kerr KM, Brambilla E, et al. EU-USA pathology panel for uniform diagnosis in randomised controlled trials for HRCT screening in lung cancer. Eur Respir J 2006;28:1186-9.
- Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Arch 2012;461:185-93.
- Thunnissen E BJ, Kerr K, Chung JH, et al. In compressed lung tissue microscopic sections of adenocarcinoma in situ may mimic papillary adenocarcinoma. Arch Pathol Lab Med 2012. [Epub ahead of print].
- Flieder DB. Screen-detected adenocarcinoma of the lung. Practical points for surgical pathologists. Am J Clin Pathol 2003;119:S39-57.
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2012. [Epub ahead of print].
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504.
- Shim HS, Lee da H, Park EJ, et al. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135:1329-34.
- Sterlacci W, Savic S, Schmid T, et al. Tissue-sparing application of the newly proposed IASLC/ATS/ERS classification of adenocarcinoma of the lung shows practical diagnostic and prognostic impact. Am J Clin Pathol 2012;137:946-56.
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46.
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64.
- Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012;25:405-15.
- Kim MJ, Shin HC, Shin KC, et al. Best immunohistochemical panel in distinguishing adenocarcinoma from squamous cell carcinoma of lung: tissue microarray assay in resected lung cancer specimens. Ann Diagn Pathol 2012. [Epub ahead of print].
- Nonaka D. A study of ΔNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol 2012;36:895-9.
- Pardo J, Martinez-Peñuela AM, Sola JJ, et al. Large cell carcinoma of the lung: an endangered species? Appl Immunohistochem Mol Morphol 2009;17:383-92.
- Rossi G, Marchioni A, Milani M, et al. TTF-1, cytokeratin 7, 34betaE12, and CD56/NCAM immunostaining in the subclassification of large cell carcinomas of the lung. Am J Clin Pathol 2004;122:884-93.
- Rossi G, Papotti M, Barbareschi M, et al. Morphology and a limited number of immunohistochemical markers may efficiently subtype non-small-cell lung cancer. J Clin Oncol 2009;27:e141-2; author reply e143-4.
- Rossi G, Pelosi G, Graziano P, et al. A reevaluation of the clinical significance of histological subtyping of non--small-cell lung carcinoma: diagnostic algorithms in the era of personalized treatments. Int J Surg Pathol 2009;17:206-18.
- Sigel CS, Moreira AL, Travis WD, et al. Subtyping of non-small cell lung carcinoma: a comparison of small biopsy and cytology specimens. J Thorac Oncol 2011;6:1849-56.
- Thunnissen E, Boers E, Heideman DA, et al. Correlation of immunohistochemical staining p63 and TTF-1 with EGFR and K-ras mutational spectrum and diagnostic reproducibility in non small cell lung carcinoma. Virchows Arch 2012. [Epub ahead of print].
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of Lung Adenocarcinoma in Resected Specimens: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch Pathol Lab Med 2012. [Epub ahead of print].
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of Lung Cancer in Small Biopsies and Cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch Pathol Lab Med 2012. [Epub ahead of print].
- Whithaus K, Fukuoka J, Prihoda TJ, et al. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med 2012;136:155-62.
- Warth A, Muley T, Herpel E, et al. Large-scale comparative analyses of immunomarkers for diagnostic subtyping of non-small-cell lung cancer biopsies. Histopathology 2012. [Epub ahead of print].