Unexpected long survival of brain oligometastatic non-small cell lung cancer (NSCLC) treated with multimodal treatment: a single-center experience and review of the literature
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death across the world (1). Over 50% of these patients present metastatic disease at diagnosis, while many others develop metastatic dissemination during their course of disease and about 20% of these patients develop brain metastases.
Median survival for patients with stage IV NSCLC treated with chemotherapy alone is between 8 and 11 months (2). Brain metastases development represents a negative prognostic factor and median survival in this subgroup of patients is 7 months (3,4).
Hellman and Weichselbaum defined the oligometastatic state as an intermediate clinical state between locoregionally confined and widespread cancer (5). Since 1995 it has been hypothesized that these patients with limited metastatic foci may experience longer survival and, in rare cases, potential cure following comprehensive tumor ablation.
In the last two decades a number of studies evaluating the role of locally ablative therapies [i.e., surgical resection (SR), and stereotactic radiosurgery, (SRS)] for limited metastatic foci have been performed (6,7). Patients with synchronous brain oligometastases represent a population that can benefit from aggressive locoregional treatments. SR, SRS and whole-brain radiotherapy (WBRT) are the main therapeutic options (8-10).
The treatment of single metastases with different localization than brain is controversial but several reports have shown longer survivals in this subgroup of patients treated with aggressive locoregional treatments (11-15).
We present in this report five cases of oligometastatic NSCLC patients experiencing unexpected long survival after aggressive locoregional treatment of their metastatic districts.
Case presentations
The median age of the patients at diagnosis was 53.6 years (range, 38–64 years). Clinical and pathological features of the patients are reported in Table 1.
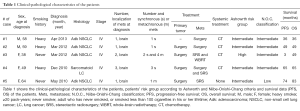
Full table
These patients have been treated at Sant’Andrea Hospital of Rome between May 2009 and April 2016. For three of them, cancer was diagnosed during the hospitalization at the Emergency Department (ED) for symptoms caused by brain metastases. These cases were immediately treated with neurosurgical intervention.
All patients had brain metastases. We defined synchronous if the metastases occurred within 2 months from the diagnosis of the primary tumor. The disease of this subgroup of patient was considered biologically more aggressive. We chose 2 months for the definition of “synchronous metastases” according to previous published data and also because of logistical concerns relative to the SEER registry process.
Cancer staging was done according to TNM 7th edition. All the patients still undergo clinical and radiological [Computed Tomography (CT) scan, brain magnetic resonance imaging (MRI)] follow-up every 6 months.
All patients are still alive, and the median overall survival (OS) is 49 months (range, 36–83 months).
Progression-free survival (PFS) was defined as the time from the first SR to the first radiological evidence of disease progression.
OS was defined as the time from diagnosis until death from any cause.
Survival data were calculated from the day of first treatment of either the primary tumor or metastatic lesions for patients with synchronous metastases, and from the day of first treatment of oligometastatic disease for patients with metachronous metastases.
Written informed consent was obtained from all the patients for publication of this case series.
Case 1
A 58-year-old, heavy smoker (45 pack-years), Caucasian male was admitted to the ED for speech impairment and tonic-clonic seizures on April 2013. A brain MRI revealed a 12 mm left fronto-subcortical lesion with associated edema. A total body CT scan showed a 10 mm nodule in the right lung. An urgent SR of the brain mass was performed. An atypical resection of the middle lobe of the right lung was performed three weeks later. The histological examinations showed a brain metastasis of lung adenocarcinoma (ADK) [TTF-1+, CK7+, EGFR wild type (wt) and rearrangement of ALK] and a chondroid hamartoma of the lung, respectively. From August 2013 to January 2015 the patient received a first line treatment with platinum and pemetrexed for 6 cycles followed by maintenance pemetrexed for a further 6 months. Since subsequent total body CT scan didn’t show evidence of disease, the patient started the follow-up with an OS of 36 months.
Case 2
On March 2012, a 59-year-old Caucasian male, heavy smoker (42 pack-years) was admitted to the ED for balance disorder. A brain MRI showed a 15 mm cerebellar lesion. An urgent cerebral surgery was performed and the histological and immunohistochemistry features suggested a metastasis of NSCLC ADK TTF-1+. A CT scan revealed a mass in the right lung and in April 2012 the patient underwent a right upper lobectomy with systematic lymphadenectomy for stage IV grade 3 lung ADK (pT2a pN0 M1b, EGFR wt, no ALK rearrangement). There was no radiological evidence of disease after surgery. From July 2012 to October 2012 the patient received 4 cycles of platinum-based chemotherapy. There is no clinical and radiological evidence of disease recurrence so far, with an OS of 49 months.
Case 3
A 38-year-old, never smoker, Caucasian female underwent a left lower lobectomy and systematic lymphadenectomy on March 2012 for stage IIIA NSCLC ADK (pT3 pN2 Mx, EGFR wt, no rearrangement of ALK and ROS-1). On April 2012 she came for a visit at our department and we decided to complete the staging with a brain MRI. The exam showed the presence of two brain metastases. Between May and September 2012, she received 6 cycles of platinum based chemotherapy and she underwent SRS of the brain metastases. On March 2013 brain MRI showed new metastases and the patient underwent SRS and whole brain radiotherapy (WBRT). At April 2016 the disease is still under control and the OS is 49 months.
Case 4
A 49-year-old Caucasian female, heavy smoker (36 pack-years) was admitted to the ED with seizures and hypoesthesia of the right arm on December 2010. A total body CT scan showed a brain lesion and a left lung mass. The patient underwent craniotomy with total excision of the brain lesion first: the histological exam revealed a metastatic NSCLC TTF-1+ CK7+. The patient subsequently underwent left upper lobectomy with systematic lymphadenectomy. Histological analysis showed a sarcomatoid lung cancer (pT2 pN0 M1b). On February 2011, a brain MRI showed three new lesions, and so she performed SRSs. From March to July 2011, she received 6 cycles of platinum based chemotherapy. On February 2012, a total body CT scan revealed an increase in dimension of the frontal brain lesion so she underwent a second cerebral surgery for mass removal. The histological examination showed necrosis and inflammations, with no evidence of cancer tissue. Afterwards, she continued the radiological and clinical follow-up. The patient is still alive and the OS is 65 months.
Case 5
A 64-year-old Caucasian female, never smoker, underwent a left lower lobectomy on May 2009 for stage IA lung ADK (pT1 pN0 G3, EGFR wt). A PET scan performed after surgery was negative. According to the stage of disease the patient did not undergo adjuvant treatment. On February 2010 a total body CT scan and a brain MRI showed the presence of one left frontal brain metastasis. The patient underwent a SRS and then she started radiological and clinical follow-up. At April 2016 the patient is still alive with no evidences of disease and the OS is 83 months.
Discussion
An increasing interest in oligometastatic condition has been observed in scientific literature since this condition for NSCLC has been defined in 1995 by Hellman and Weichselbaum (5). Many reports demonstrate that this subgroup of patients may experience a long survival or even healing (16,17). Nevertheless, only one clinical randomized trial has been published so far, and the management remains controversial. However, local therapies aimed to eradicate the disease seem to be the most reliable strategies (16).
A recent systematic review published by Ashworth and colleagues, analyzing 49 reports and 2,176 oligometastatic NSCLC patients, showed highly variable results in terms of survival (18). In fact, in this report, OS ranges between 5.9 and 52 months (median 14.8 months) and time to progression (TTP) from 4.5 to 23.7 months (median 12 months). In particular, they observed a longer survival (19 months) in patients with the primary tumor controlled by local treatments. The longest survival has been reported in patients with mixed metastatic sites, while patients with adrenal gland metastases showed the shortest survival (18).
The same group of researchers published in 2014 an individual patient data metanalysis, on 757 NSCLC patients with 1–5 metastases (19). Both primary tumor and metastatic sites were treated with surgery. Twenty six months of median OS and 11 months of median PFS were observed. The authors divided the patients into three classes of prognosis: low risk, including patients with metachronous metastases; intermediate risk, including patients with synchronous metastases and no nodal involvement; high risk, including patients with synchronous metastases and nodal involvement (N1–N2). The 5-year OS were 47.8%, 36.2% and 13.8%, respectively. Other prognostic factors were: primary tumor (T) stage; histology (better prognosis for ADK); presence of brain or lung metastases and surgical treatment of the T (19).
Niibe and colleagues proposed another classification (Niibe-Onishi-Chang classification) for oligometastatic patients in 2013. This classification divided the patients in favorable, intermediate and unfavorable prognosis, according to the timing of metastases occurrence (synchronous or metachronous), the number and the location of metastatic sites (20).
Although the traditional management for metastatic NSCLC contemplates only palliative treatments, recent data show that surgery of the primary tumor leads to a better survival in patients with NSCLC and brain metastases (8,17,21,22).
Iwasaki and colleagues demonstrated that patients that underwent lung and brain surgery experience a longer survival than patients who underwent resection of the lung lesion only (23). American College of Chest Physicians (ACCP) Guidelines suggest SR of the brain metastasis followed by WBRT and adjuvant chemotherapy as standard of care for patients with a single brain metastatic focus (24).
The management of multiple brain metastases is more controversial. SRS may be the preferable option in these cases (25).
Interestingly, recent data, mainly reported as case reports, showed a tumor regression at non-irradiated sites. This effect, called “abscopal”, could be mediated by immune system, by enhancing T cells function, inducing immunogenic cells’ death and improving antigen expression and presentation (26,27).
The concern in understanding the biology of oligometastatic cancer is lately increasing. It is commonly accepted that metastatic progression is a multi-step process. Two hypotheses to explain the differences between oligometastases and polymetastases have been proposed. According to the first hypothesis oligometastases and polymetastases may have different phenotypes, determined by clonal populations with different metastatic potential. The second hypothesis suggests that metastatic process may be a continuum and oligometastatic disease is an early phase that can evolve in polymetastatic stage (28).
In this report we present five cases of oligometastatic lung cancer, showing a long survival, after a multimodal treatment. In our cases the PFS ranges between 3 to 65 months. All patients are still alive and progression free, with an OS ranging between 36 and 84 months. All patients underwent surgery for the primary tumor.
Brain metastases were synchronous in four cases and in three of these were symptomatic (i.e., seizure, speech impairments, arm hypoesthesia and balance disorders). SR was performed in these three cases in order to relieve symptoms, while SRS alone or in association with WBRT was the selected therapeutic option for asymptomatic lesions. Only one patient with multiple symptomatic brain metastases received both surgical and radiation treatment. None received WBRT after surgery. The PFS and OS observed are surprisingly longer than those reported in the literature. These data are showed not only in patients with single metastasis and despite the classification of risk and prognosis according to Ashworth and Niibe-Onishi-Chang. In fact, only one of the cases we report is classified as poor risk or favorable prognosis (Table 1).
We performed a review of the last 5 years’ literature published on Medline (via PubMed—last search performed on June 2016). We considered all the reports presenting data about oligometastatic NSCLC patients with brain metastases. We excluded the studies enrolling patients with various tumor types (8,11-13,27,29-34). As reported in Table 2, literature data are available from case reports, retrospective series and phase II trials. Very heterogeneous outcomes are reported, with OS ranging from 11.2 to over 68 months. As expected, the better outcomes were described in case reports than in larger studies. Two retrospective series enrolled only patients with brain metastases. Nevertheless, they shown conflicting results, with a long OS of 31.8 months in Arrieta and colleagues’ series and a shorter OS (11.2 months) in Enders and colleagues’ report (12,30). A retrospective series published by Fleckenstein and colleagues addresses the relevance of synchronous vs. metachronous metastasis, not founding a prognostic relevance in this characteristic (33).
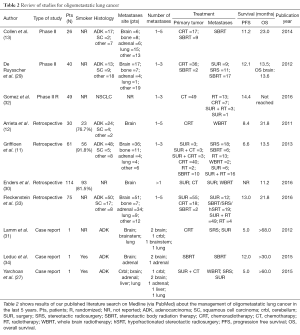
Full table
Interestingly, during the 2016 ASCO annual meeting the first randomized trial on oligometastatic NSCLC was presented. In this phase II study patients randomized to receive locoregional treatments showed a better PFS (14.4 months) than the control group (PFS 3.9 months) (32). Furthermore, treatment options are very heterogeneous, both for primary tumor and metastatic sites.
Since few data from randomized or homogeneous trials are currently available in literature, a growing interest in oligometastatic disease is observed. An increasing number of trials designed in order to study the effectiveness of local treatments (radiotherapy or surgery) are ongoing (Table 3). In particular, a phase III trial studying the best timing for chemotherapy and chemoradiotherapy (CRT) is ongoing (NCT02076477), and another phase III trial comparing chemotherapy alone vs. chemotherapy and stereotactic ablative radiotherapy (SABR) is open, but is not yet recruiting (NCT02417662).
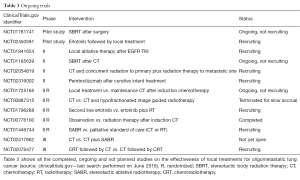
Full table
Recent advances demonstrate a correlation between microRNA expression and oligometastatic stage (28,35,36). Lussier and colleagues identified certain microRNAs associated with a high rate of progression (HRP) and others with a low rate of progression (LRP) in NSCLC patients (36). Circulating tumor cells in oligometastatic patients are under investigation as well (NCT02949837). Further analyses are required. In fact, a better knowledge of tumor biology can help oncologists to identify patients with indolent disease, who could benefit from local treatment.
Conclusions
This case series confirms that oligometastatic NSCLC patients treated with locoregional aggressive therapies experience better outcomes and longer survivals. In fact, despite the evidence that stage IV NSCLC can benefit only from palliative oncologic therapies, curative radiotherapy and surgery of the metastatic loci turned out to be a potential better therapeutic option for this subgroup of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13 Suppl 1:5-13. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [PubMed]
- Timmerman RD, Bizekis CS, Pass HI, et al. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin 2009;59:145-70. [Crossref] [PubMed]
- Timmerman RD, Kavanagh BD. Stereotactic body radiation therapy. Curr Probl Cancer 2005;29:120-57. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 2014;25:1954-9. [Crossref] [PubMed]
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol 2008;98:202-6. [Crossref] [PubMed]
- Villarreal-Garza C, de la Mata D, Zavala DG, et al. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer 2013;14:6-13. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Niibe Y, Chang JY, Onishi H, et al. Oligometastases/Oligo-recurrence of lung cancer. Pulm Med 2013;2013:438236.
- Modi A, Vohra HA, Weeden DF. Does surgery for primary non-small cell lung cancer and cerebral metastasis have any impact on survival? Interact Cardiovasc Thorac Surg 2009;8:467-73. [Crossref] [PubMed]
- Moazami N, Rice TW, Rybicki LA, et al. Stage III non-small cell lung cancer and metachronous brain metastases. J Thorac Cardiovasc Surg 2002;124:113-22. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg 2004;26:488-93. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol 2009;92:275-82. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Yarchoan M, Lim M, Brahmer JR, et al. Oligometastatic Adenocarcinoma of the Lung: A Therapeutic Opportunity for Long-Term Survival. Cureus 2015;7:e409. [PubMed]
- Uppal A, Ferguson MK, Posner MC, et al. Towards a molecular basis of oligometastatic disease: potential role of micro-RNAs. Clin Exp Metastasis 2014;31:735-48. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Enders F, Geisenberger C, Jungk C, et al. Prognostic factors and long-term survival in surgically treated brain metastases from non-small cell lung cancer. Clin Neurol Neurosurg 2016;142:72-80. [Crossref] [PubMed]
- Lamm AF, Elaimy AL, Mackay AR, et al. Long-Term Survival of a Patient with Brainstem and Recurrent Brain Metastasis from Stage IV Nonsmall Cell Lung Cancer Treated with Multiple Gamma Knife Radiosurgeries and Craniotomies: A Case Report and Review of the Literature. Case Rep Oncol Med 2012;2012:621641.
- Gomez DR, Blumenschein GR, Lee JJ, et al. Local consolidative therapy (LCT) to improve progression-free survival (PFS) in patients with oligometastatic non-small cell lung cancer (NSCLC) who receive induction systemic therapy (IST): Results of a multi-institutional phase II randomized study. J Clin Oncol 2016;34:abstr 9004.
- Fleckenstein J, Petroff A, Schäfers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016;16:348. [Crossref] [PubMed]
- Leduc C, Antoni D, Quoix É, et al. Stereotactic body radiation radiotherapy for oligometastatic non-small cell lung cancer (NSCLC): A case report. Cancer Radiother 2015;19:192-7. [Crossref] [PubMed]
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One 2011;6:e28650. [Crossref] [PubMed]
- Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One 2012;7:e50141. [Crossref] [PubMed]


